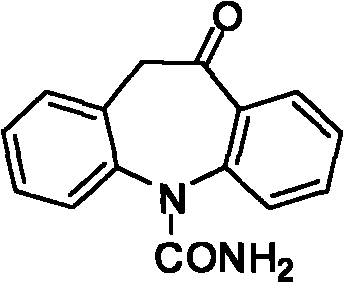
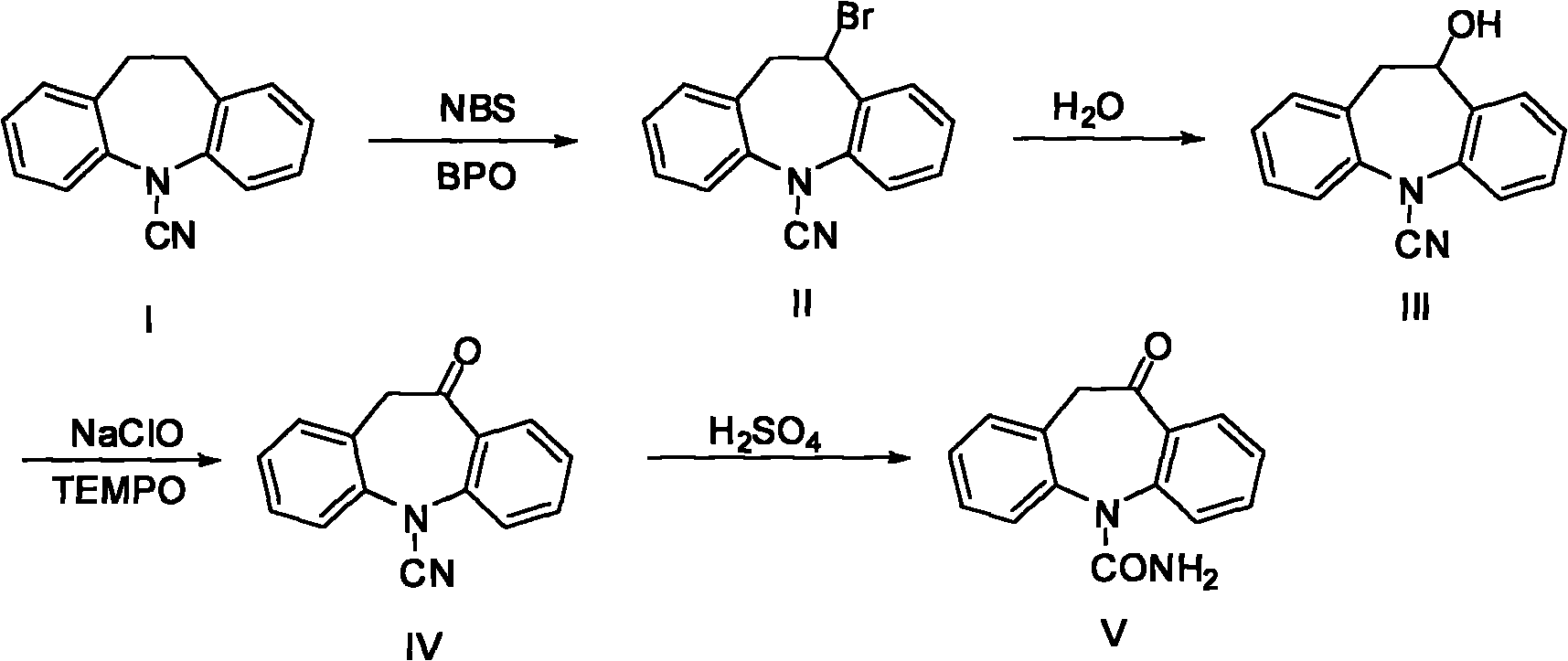
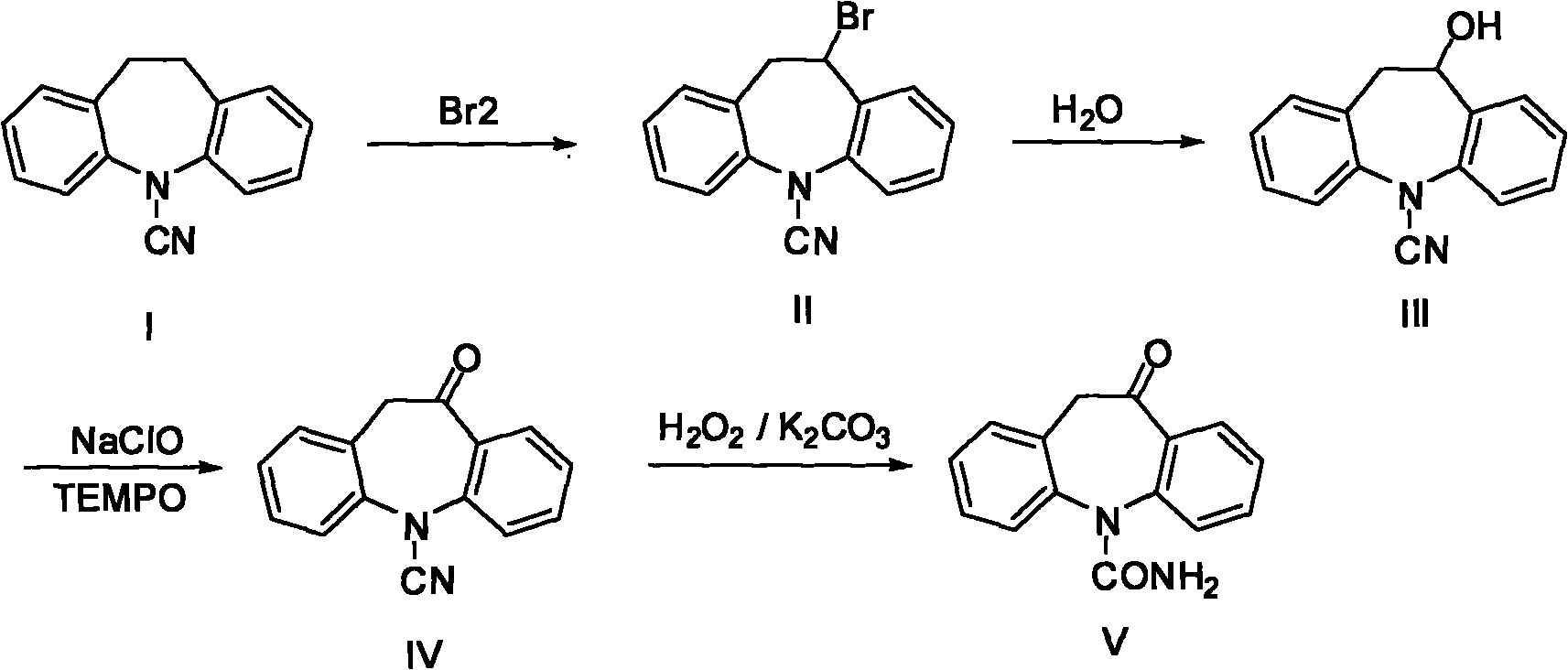
Chemical synthetic method of azepine derivate
A synthesis method and azepine technology are applied in the field of chemical synthesis of azepine derivatives, can solve the problems of large amount of sulfuric acid waste water, cumbersome treatment process, difficult recovery of acetic acid, etc., and achieve reasonable process conditions, simple and quick operation, and reaction Productivity improvement effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Preparation of Example 1.5-cyano-10-bromo-10,11-dihydro-5H-diphenyl[b,f]azepine (II)
[0026] 10g of 5-cyano-10,11-dihydro-5H-diphenyl[b,f]azepine (I), 60mL of chlorobenzene was stirred and dissolved, and 10mL of purified water was added, the temperature was raised to 85°C, and the solution was slowly added dropwise A mixture of bromine 8g / 60mL chlorobenzene, the temperature is controlled at 85-90°C, after 8 hours of dripping, continue to keep warm for 0.5 hours. After the reaction, the reaction solution was allowed to stand for stratification, and the temperature was controlled not lower than 50°C to avoid solid precipitation. The organic phase was taken, and 60 mL of solvent was evaporated under reduced pressure, and the solid was precipitated by cooling, recrystallized from acetone to obtain a yellow solid 5-cyanide 11.8 g of 10-bromo-10,11-dihydro-5H-diphenyl[b,f]azepine (II). (HPLC: 97.83%), yield 87%.
Embodiment 2
[0027] Preparation of Example 2.5-cyano-10-bromo-10,11-dihydro-5H-diphenyl[b,f]azepine (II)
[0028] 10g (I), 60mL of chlorobenzene were stirred and dissolved, and 8g Na 2 CO 3 , the temperature was raised to 85°C, and a mixture of liquid bromine 8g / 60mL chlorobenzene was slowly added dropwise, the temperature was controlled at 95°C, the dripping was completed after 7h, and the temperature was continued for 0.5h. After the reaction, filter while hot, rinse the filter cake with 10mL of chlorobenzene, evaporate 60mL of solvent from the reaction solution under reduced pressure, cool and precipitate a solid, and recrystallize from acetone to obtain a yellow solid 5-cyano-10-bromo-10,11-di Hydrogen-5H-diphenyl[b,f]azepine(II) 10.9g (HPLC: 97.51%), yield 80%.
Embodiment 3
[0029] Preparation of Example 3.5-cyano-10-bromo-10,11-dihydro-5H-diphenyl[b,f]azepine (II)
[0030] Dissolve 10g (I) and 60mL dichloroethane with stirring, add 8g Na 2 CO 3 , the temperature was raised to 85°C, and a mixture of 8g of liquid bromine / 60mL of dichloroethane was slowly added dropwise, under total reflux, the temperature was at 83°C, after 7h of dropping, the temperature was continued for 0.5h. After the reaction, filter while hot, rinse the filter cake with 10mL of dichloroethane, distill off 60mL of solvent from the reaction solution under reduced pressure, cool to precipitate a solid, and recrystallize from acetone to obtain a yellow solid 5-cyano-10-bromo-10,11 -Dihydro-5H-diphenyl[b,f]azepine (II) 11.1 g (HPLC: 98.12%), yield 82%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com