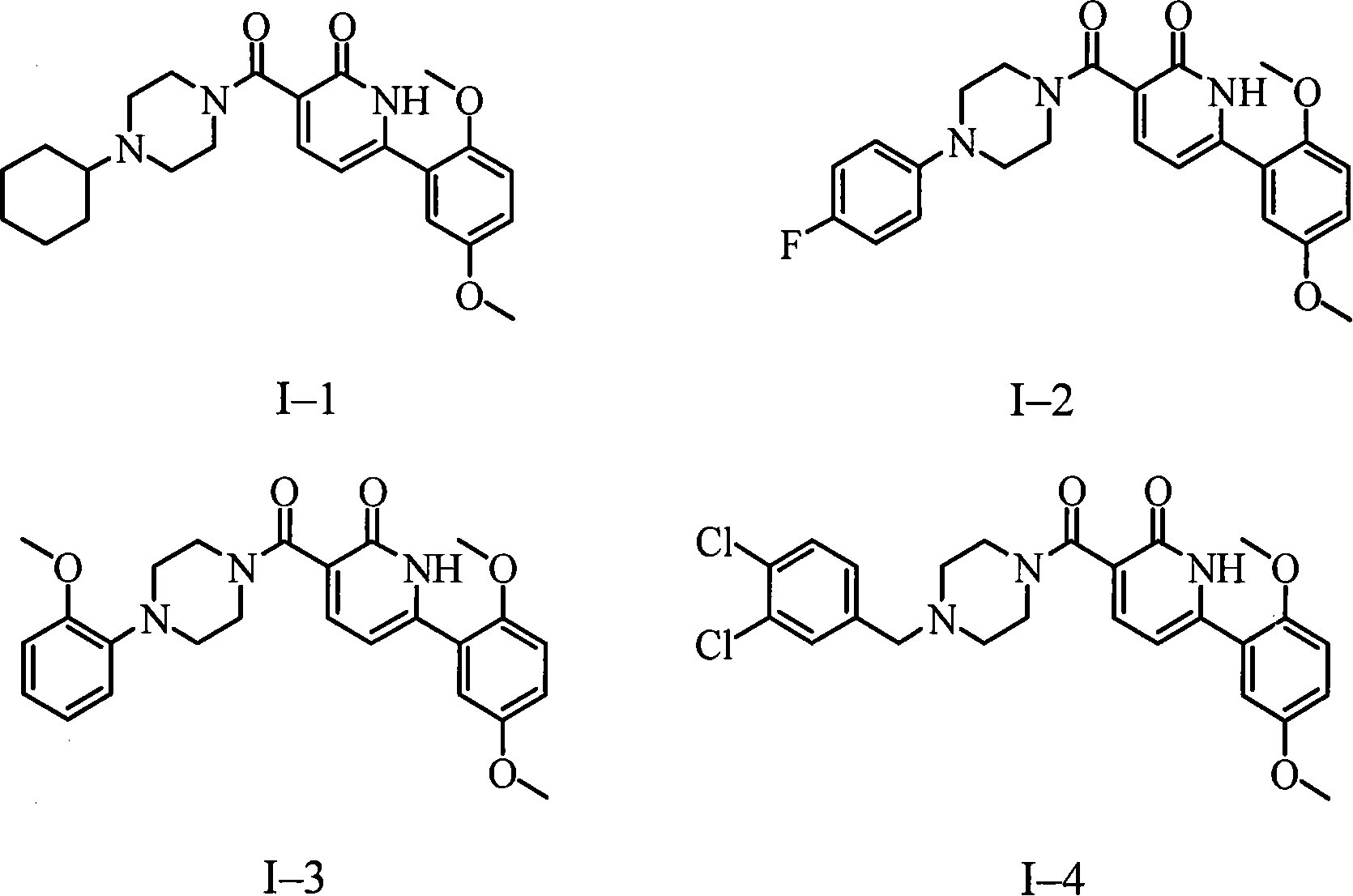
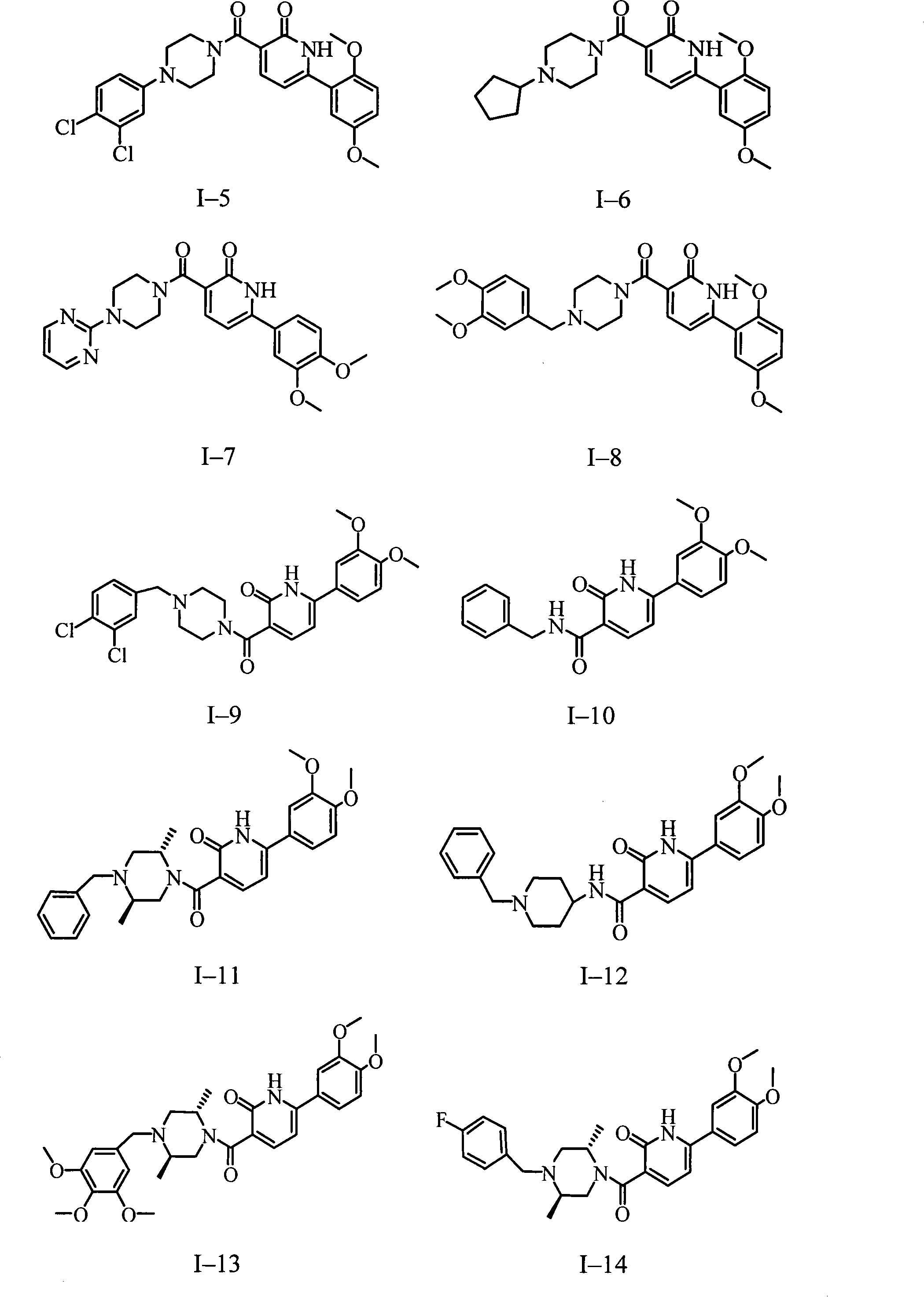
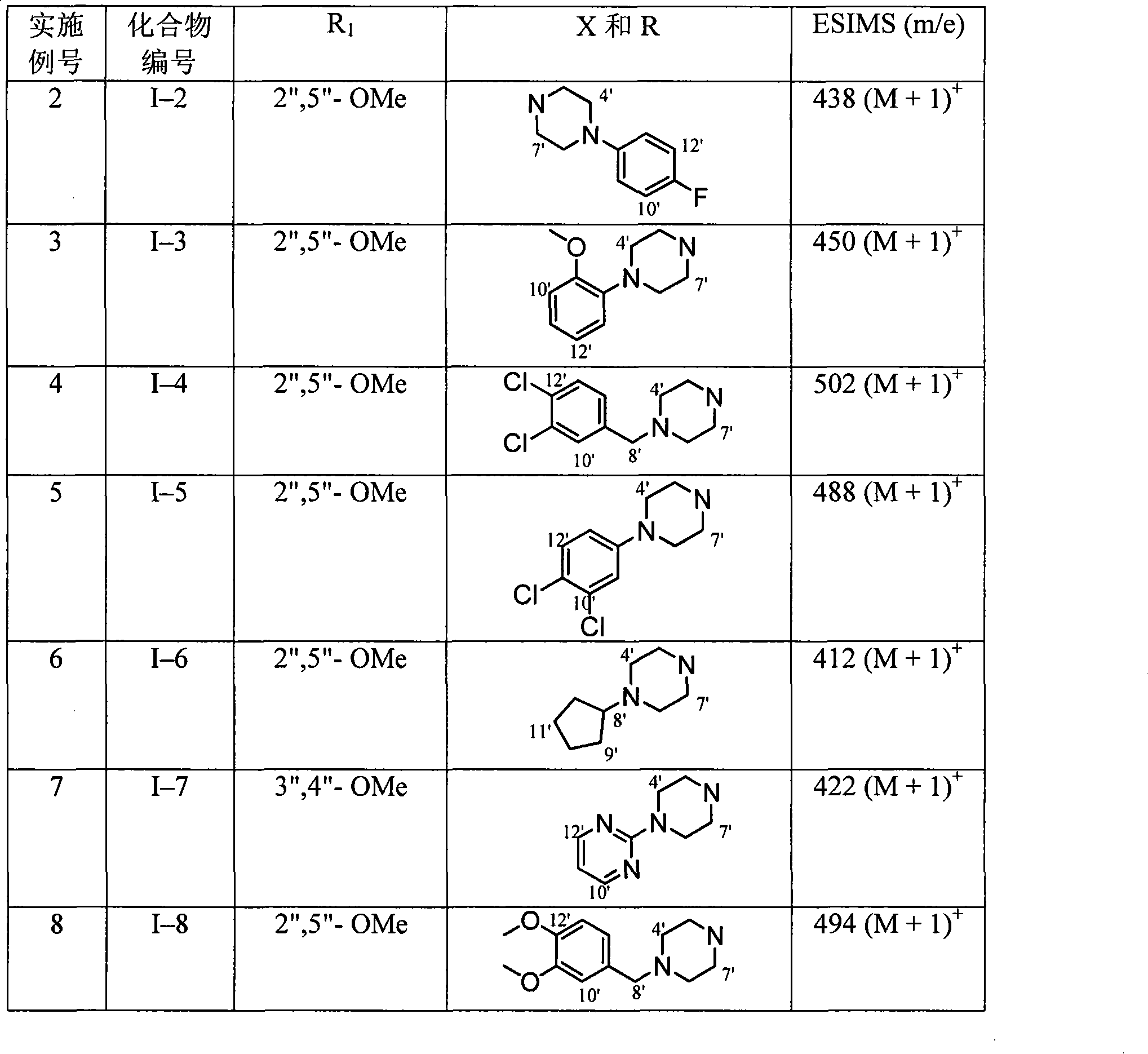
Use of aryl-3-substituted carbonyl pyridone compound
A technology of pyridone and compound, applied in the direction of active ingredients of heterocyclic compounds, drug combinations, antineoplastic drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Preparation of starting material A (3,4-dimethoxyacetophenone):
[0026] starting material A
[0027] Catechol (11.0 grams, 0.1 moles) was dissolved in 150 milliliters of acetone, potassium carbonate (27.6 grams, 0.20 moles) and dimethyl sulfate (12.6 grams, 0.1 moles) were added; reflux reaction for 10 hours, TLC showed After the reaction was complete, it was filtered, the filter cake was washed with ethyl acetate, and concentrated to obtain a crude oil product, which was subjected to short silica gel column chromatography to obtain o-dimethoxybenzene (12.1 g, yield 81%).
[0028] O-dimethoxybenzene (13.8 grams, 0.1 moles) was dissolved in 150 milliliters of dichloromethane, then anhydrous zinc chloride powder (26.8 grams, 0.20 moles) was added, and acetic anhydride (15.3 g, 0.15 moles); after the dropwise addition, the reaction was slowly raised to room temperature for 12 hours, then the reactant was carefully poured into 600 milliliters of ice water, e...
Embodiment 2
[0029] Example 2: Preparation of starting material B (2,5-dimethoxyacetophenone):
[0030] starting material B
[0031]In the same manner as in Example 1, using p-diphenol (11.0 g, 0.1 mol) as a raw material, the starting material B (2,5-dimethoxyacetophenone) was obtained as a colorless oil. H NMR spectrum 1 H-NMR (400MHz, deuterated chloroform, δppm) 2.62 (single peak, 3H, COCH 3 ), 3.77 (single peak, 3H, OCH 3 ), 3.79 (single peak, 3H, OCH 3 ), 6.89 (doublet, 1H, J=8.4Hz, H-4), 7.03 (doublet, 1H, J=8.4Hz, H-3), 7.30 (singlet, 1H, H-6).
Embodiment 3
[0032] Example 3: Preparation of intermediate compound IIIa [3-cyano-6-(3,4-dimethoxyphenyl)-2H-pyridin-2-one]:
[0033] Compound IIIa
[0034] Sodium metal (2.76 g, 120 mmol) was added to 250 ml of ether, 1 ml of ethanol was added dropwise, and starter A (3,4-dimethoxyacetophenone) (100 mmol ) and ethyl formate (150 mmol) mixture, after the dropwise addition, the mixture was stirred for 15 minutes, then warmed up to room temperature and reacted for 1 hour, after diethyl ether was evaporated under reduced pressure, the solid mixture was added cyanoacetamide (12.6 grams, mol) and water (400 ml). After the mixture was refluxed for 8 hours, cooled, acidified with acetic acid, and filtered to obtain a yellow solid, after drying, the initial product was recrystallized from ethanol to obtain intermediate compound IIIa[3-cyano-6-(3,4-dimethoxy Phenyl)-2H-pyridin-2-one]: Yield: 56%, pale yellow solid; Melting point > 250°C; R f (Dichloromethane / methanol 20:1) 0.46; H NMR 1 H-N...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com