Xinshuning tablets quality control method
A quality control method, the technology of Xinshuning Tablets, is applied in the field of quality control of Xinshuning Tablets, which can solve the problems of less identification of medicinal flavors and inability to effectively control product quality, so as to ensure safety and effectiveness, improve specificity and stabilize quality sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0026] The present invention will be further described below in conjunction with embodiment.
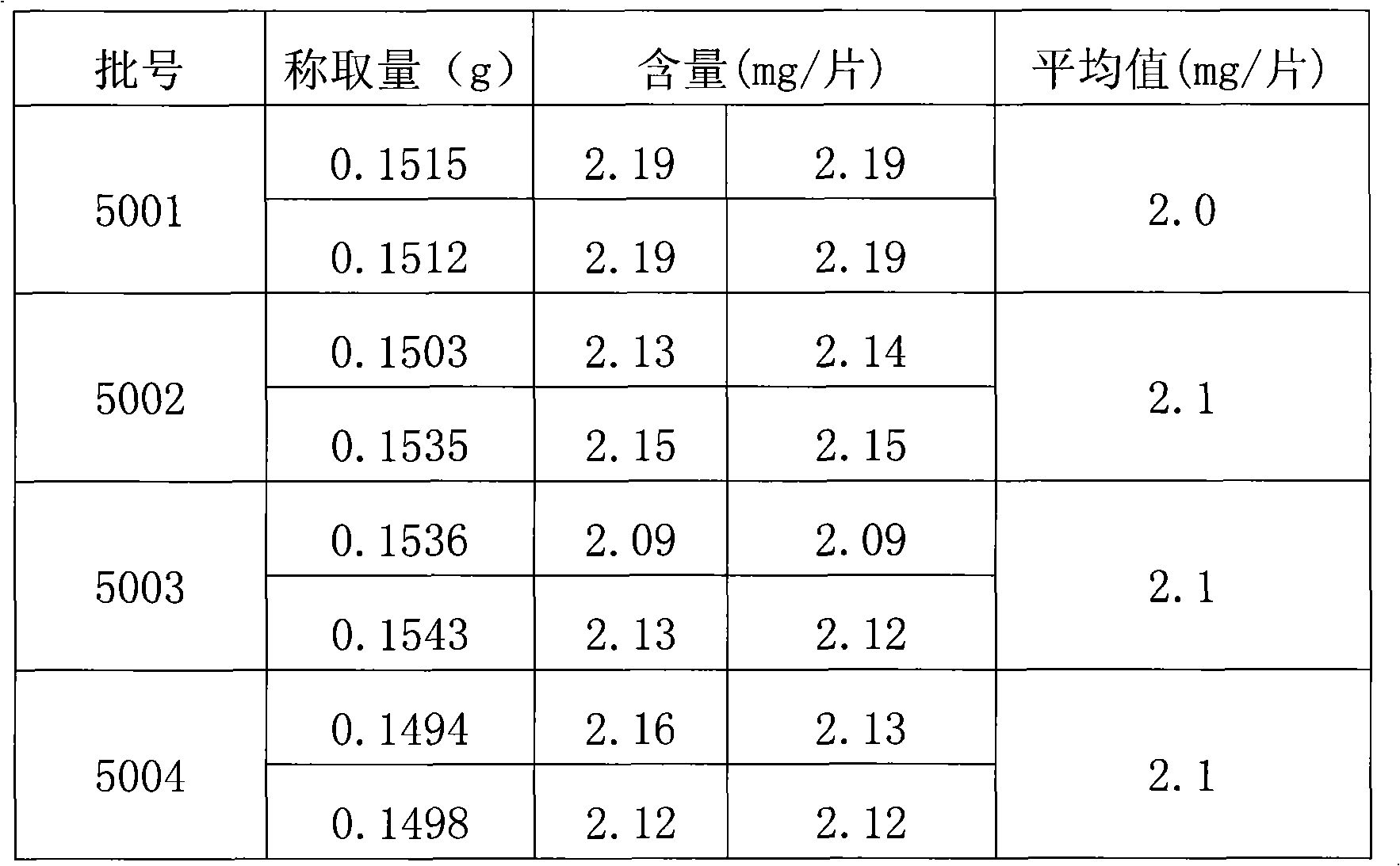
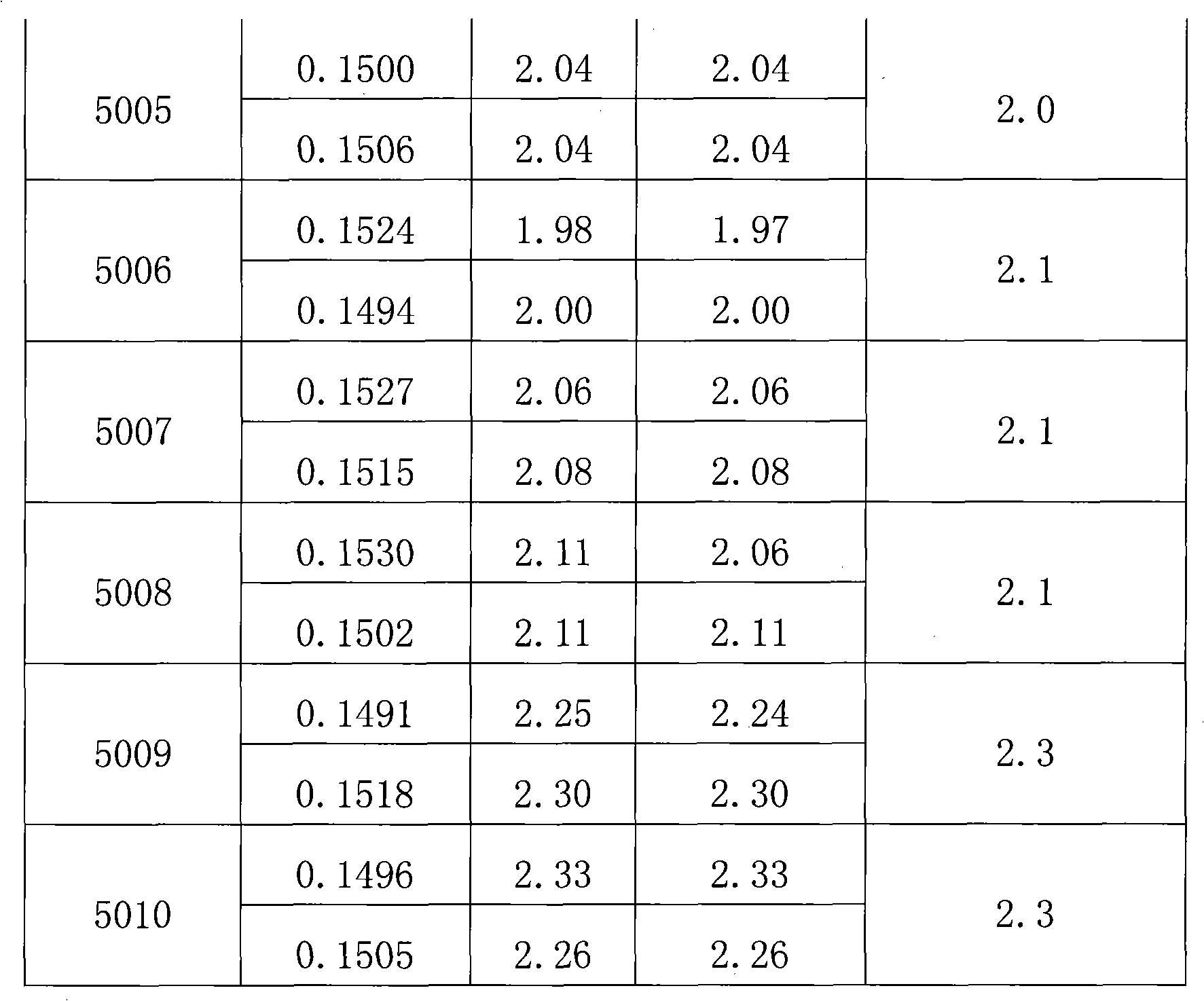
[0027] Get the perhexiline tablet that the batch number produced by the applicant is 5001.
[0028] [Identification] (1) Take 2 tablets of this product, remove the sugar coating, grind finely, add 10ml of methanol, ultrasonically treat for 15 minutes, filter, and the filtrate is used as the test solution. Another 1 g of ginkgo biloba reference drug was taken, and the reference drug solution was prepared in the same way. Test according to thin-layer chromatography (Appendix VI B of Chinese Pharmacopoeia 2005 Edition), draw 5 μl of each of the above two solutions, and place them on the same silica gel G thin-layer plate containing 4% sodium acetate respectively, and use ethyl acetate-butanone -Formic acid-water (5:3:1:1) was used as the developer, developed, taken out, dried in the air, and inspected under ultraviolet light (365nm). In the chromatogram of the test product, there are ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com