Cyclohexene derivative or pharmaceutically acceptable salt thereof and application of cyclohexene derivative and salt
A technology of cyclohexene and derivatives, applied in the field of medicine, can solve problems such as difficult to meet clinical needs, increase dosage, and decrease activity, and achieve the effects of meeting the needs of clinical applications, improving stability, and good inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Preparation of (3R,4R,5S)-4-acetamido-5-guanidino-3-((3R)-3-n-butanesulfonamidopiperidine)-1-cyclohexene-1-carboxylic acid.
[0055] a) (3R,4S,5S)-4-acetamido-5-azido-3-((3R)-3-n-butylsulfonamide piperidine)-1-cyclohexene-1-carboxylic acid ethyl Preparation of esters.
[0056]
[0057] Preparation of (3R,4R,5S)-4-acetamido-5-azido-3-acetoxy-1-cyclohexene-1-carboxylic acid ethyl ester: See J.Am.Chem.Soc for the preparation method ., 1997(119):681-690.
[0058] Place 20mmol of (3R,4R,5S)-4-acetamido-5-azido-3-acetoxy-1-cyclohexene-1-carboxylic acid ethyl ester and 1mmol of tetrakistriphenylphosphine palladium in In a dry two-necked flask, replace the air in the system twice with nitrogen, add 40mL redistilled DMF with a syringe and stir evenly, add 40mmol DIPEA (N,N-diisopropylethylamine) and stir to cool to 0°C , slowly add (R)-3-n-butylsulfonamidopiperidine trifluoroacetate DMF (40mL) solution dropwise, continue to stir at 0°C for 20 minutes after the addition, tr...
Embodiment 2
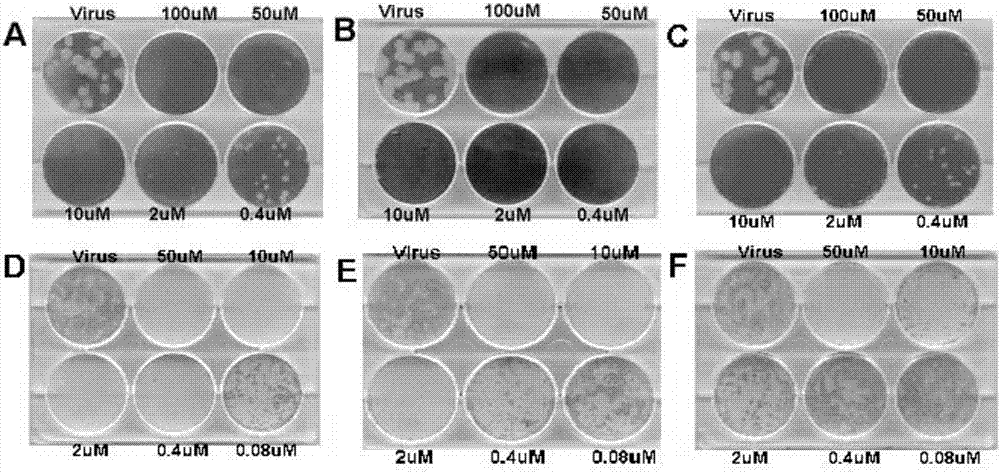
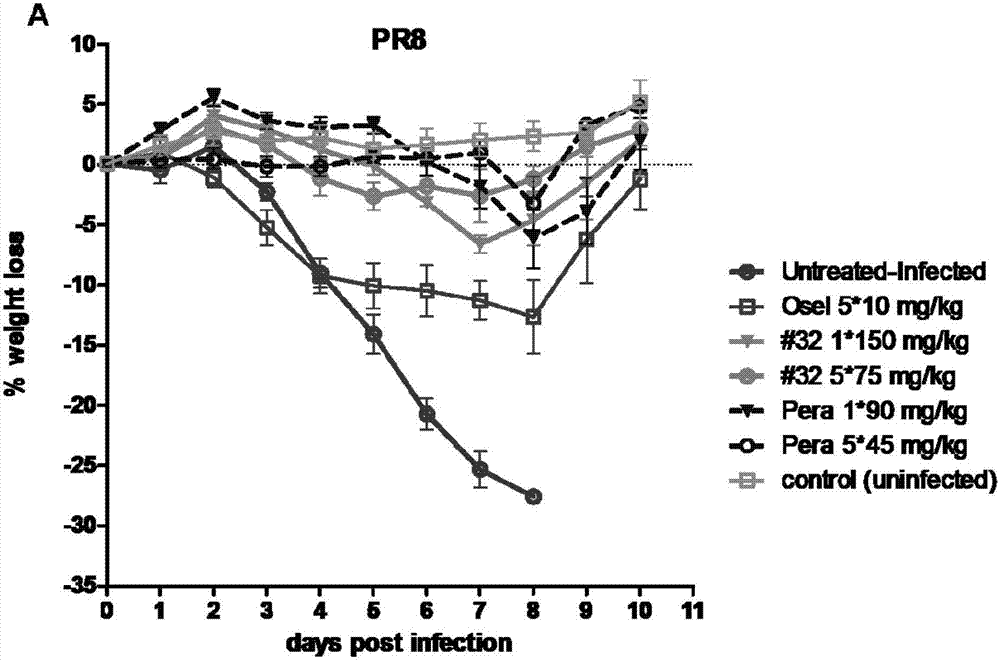
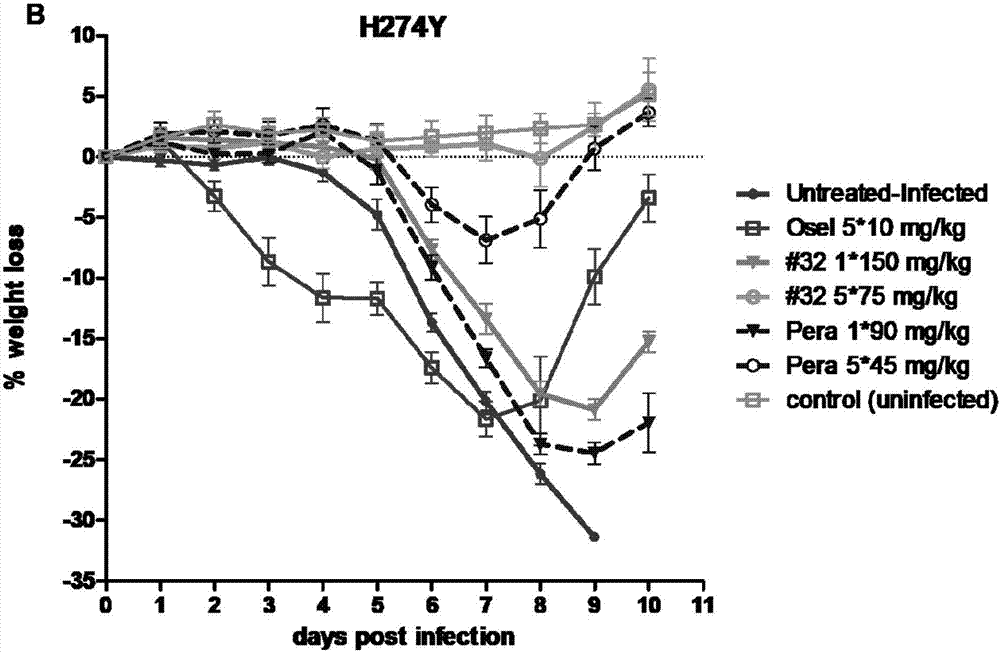
[0088] 1. Determination of Influenza Virus Neuraminidase Activity
[0089] Experimental materials: NA enzyme (neuraminidase) liquid: the allantoic fluid of chicken embryos infected with influenza virus
[0090] Enzymatic reaction system: 330mmol / L MES buffer (pH3.5)
[0091] 200μmol / L fluorescent substrate MUNANA
[0092] 4mmol / L CaCl 2 the solution
[0093] Stop solution: 14mmol / L NaOH solution (14mmol / L, 83% ethanol)
[0094] Oseltamivir: 1mmol / L
[0095] Peramivir: 1mmol / L
[0096] Reference compound: the following compound of formula II, 1mmol / L
[0097]
[0099]
[0100] EX=355nmXM=460nm
[0101] Note: In order to verify whether the enzyme reaction system is normal, the kinetic curve of the fluorescence value can be measured without adding stop solution.
[0102] NA inhibitor screening experiment operation:
[0103] 1. Dilute oseltamivir, peramivir or the test compound (compound of formula I, II) at 1:10, 1:100, 1:1000, 1:10...
Embodiment 3
[0142] 1. Solubility experiments of different salts of the compound of formula I.
[0143] Adopt different acids such as lactic acid, hydrochloric acid, phosphoric acid, acetic acid, malic acid, citric acid, aspartic acid and formula I compound salt-forming reaction, investigate the solubility of the different salts of formula I compound, the results are shown in the table below.
[0144] Table 3. Solubility of different salts of compounds of formula I
[0145] compound type
[0146] As can be seen from the above results, the solubility of the compound of formula I after salting with acids such as lactic acid, hydrochloric acid, phosphoric acid, acetic acid, malic acid, citric acid, and aspartic acid all increases significantly, and most of them are under the condition of pH=3.8. The solubility is greater than 100mg / mL, and when the molar ratio with lactic acid is about 1:2, the solubility of salt formation reaches 194mg / mL, and the pH of the solution is 3.8; when th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com