Polymer electrolyte membrane comprising coordination polymer
A technology of coordination polymer and electrolyte membrane, applied in solid electrolyte fuel cells, fuel cell additives, fuel cell components, etc., can solve problems such as blocking anode catalysts and power reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] polymer synthesis
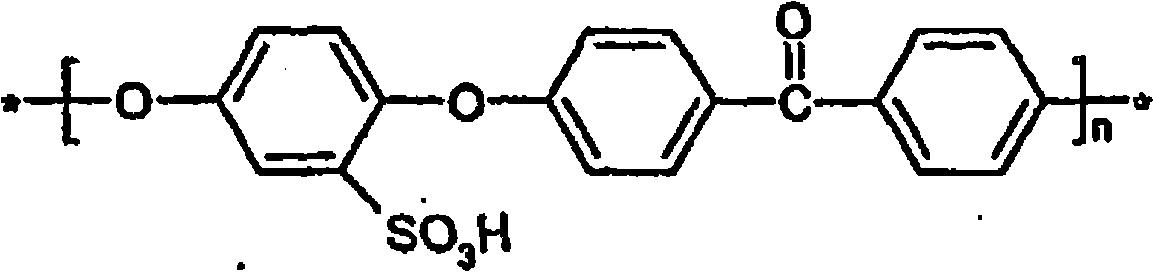
[0051] Sulfonated poly(ether ether ketone) (SPEEK) with a sulfonation degree of 51% (ion exchange capacity 1.57meq / g) was prepared using Wijers M.C., Supported liquid membranes for removal of heavy metals: permeability, selectivity and stability.Dissertation, University of Twente, The Netherlands, 1996 described the method. The poly(ether ether ketone) supplied as Victrex granules was dried under vacuum overnight at 90°C. 20 g of polymer were then dissolved in 1 liter of concentrated sulfuric acid (95% to 98%) and stirred at room temperature for 45 hours. followed by K 2 CO 3 The above polymer solution was precipitated under mechanical stirring in ice water until the pH was neutral. The precipitated polymer was left overnight. The precipitated polymer was then filtered and washed several times with distilled water and dried at 80° C. for 12 hours. The degree of sulfonation is determined by elemental analysis, e.g. Nolte R., Ledjeff K., Bauer M....
Embodiment 2
[0057] Synthesis of MOFs
[0058] Synthetic MOF(Cu 3 (BTC) 2 (H 2 O) 3 .xH 2 O), such as Schlichte K., Kratzke T., Kaskel S., Improved synthesis, thermal stability and catalytic properties of the metal-organic framework compound Cu 3 (BTC) 2 , Microporous and Mesoporous Materials 73 (2004), 81-88 described. 0.857g (3.6mmol) Cu(NO 3 ) 2 .3H 2 O was dissolved in 12 ml deionized water and mixed with 0.42 g (2.0 mmol) trimesic acid dissolved in 12 ml methanol. The solution was added to a 40 ml Teflon container placed in an autoclave and heated at 120° C. for 12 hours. The synthesis temperature (120°C) makes Cu 2 The formation of O is suppressed because Cu is avoided 2+ reduction of ions. The MOFs were characterized by nitrogen physisorption and X-ray diffractometer. Nitrogen physisorption measurements were performed at 77K using a microcrystallography ASAP 2000 instrument. X-ray powder diffraction patterns were taken with a STOE diffractometer equipped with a positi...
Embodiment 3
[0060] Composite membrane preparation
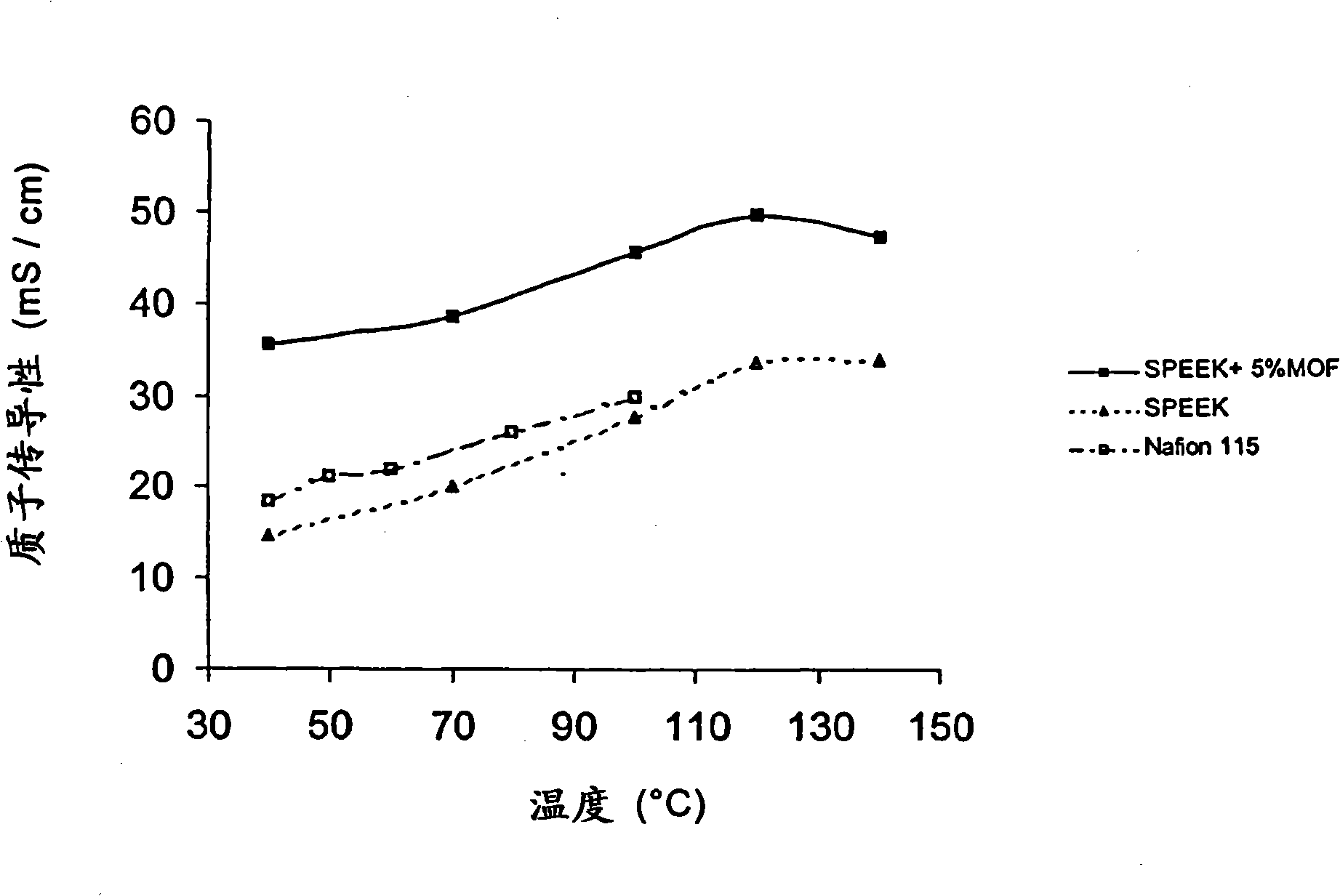
[0061] 2.3 g of polymer were dissolved in 33 g of dimethylsulfoxide (7% by weight). Then 0.12 g of MOF (5% by weight, expressed as weight of MOF / (weight of polymer and MOF) x 100%) was added to the polymer solution. The solution was stirred for 6 hours and poured on a glass plate at 60°C to remove the solvent. The glass plates were previously hydrophobized with octadecyltrichlorosilane. After casting, the SPEEK film containing 5% MOF was dried in a vacuum oven at 80 °C for 10 hours. The final thickness of the film was 96 microns. The sulfonated composite membrane was converted to its acid form by immersing the cast membrane in 2N sulfuric acid at room temperature for 24 hours, followed by two dips in water for 24 hours to ensure complete washing of residual sulfuric acid .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com