Composition for treating autonomic nerve disorder, preparation and uses thereof
A composition and drug technology, applied in the directions of drug combinations, steroids, nervous system diseases, etc., can solve problems such as poor water solubility, and achieve the effects of low cost, easy operation, and enhanced drug efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] 1. Take 1kg of commercially available oryzanol raw material (purity is 70-75%"), use 5 times the amount of ethyl acetate and absolute ethanol mixed solution with a ratio of 20:1, reflux to dissolve, filter, stand still, precipitate crystals and filter, Drying. The dried crystals (purity is about 84-88%) are reconstituted with 8 times the amount of ethyl acetate, filtered, left to stand, crystals are precipitated, filtered, and dried. The above recrystallization steps are repeated 3-5 times.
[0043] 2. Combine and recover the mother liquor filtered out after recrystallization in step 1 until the volume is 2 times that of the contained solute, let stand, precipitate crystals, filter, and dry.
[0044] 3. The crystallization (purity is about 85-87%) in step 2 is refluxed and dissolved with 8 times the amount of ethyl acetate, filtered, allowed to stand, crystals are precipitated, filtered, and dried. Repeat the above recrystallization step 3-5 times.
[0045] 4. Combine ...
Embodiment 2
[0050] 1. Chromatographic conditions and system suitability test: octadecylsilane bonded silica gel is used as filler; methanol-acetonitrile (40:60) is used as mobile phase; flow rate is 1.5ml / min; detection wavelength is 327nm; sensitivity is 0.005 AUFS. The number of theoretical plates should not be less than 2000 according to the calculation.
[0051] 2. Preparation of the test solution: Accurately weigh 10 mg of the commercially available oryzanol raw material and 10 mg of the raw material of the present invention, put it in a 10 ml measuring bottle, add water to dissolve and constant volume, and shake well to obtain the test solution.
[0052] 3. Determination: Accurately draw 10 μl of the test solution, inject it into a liquid chromatograph, record the chromatogram, and check the percentage content of each component by the area normalization method.
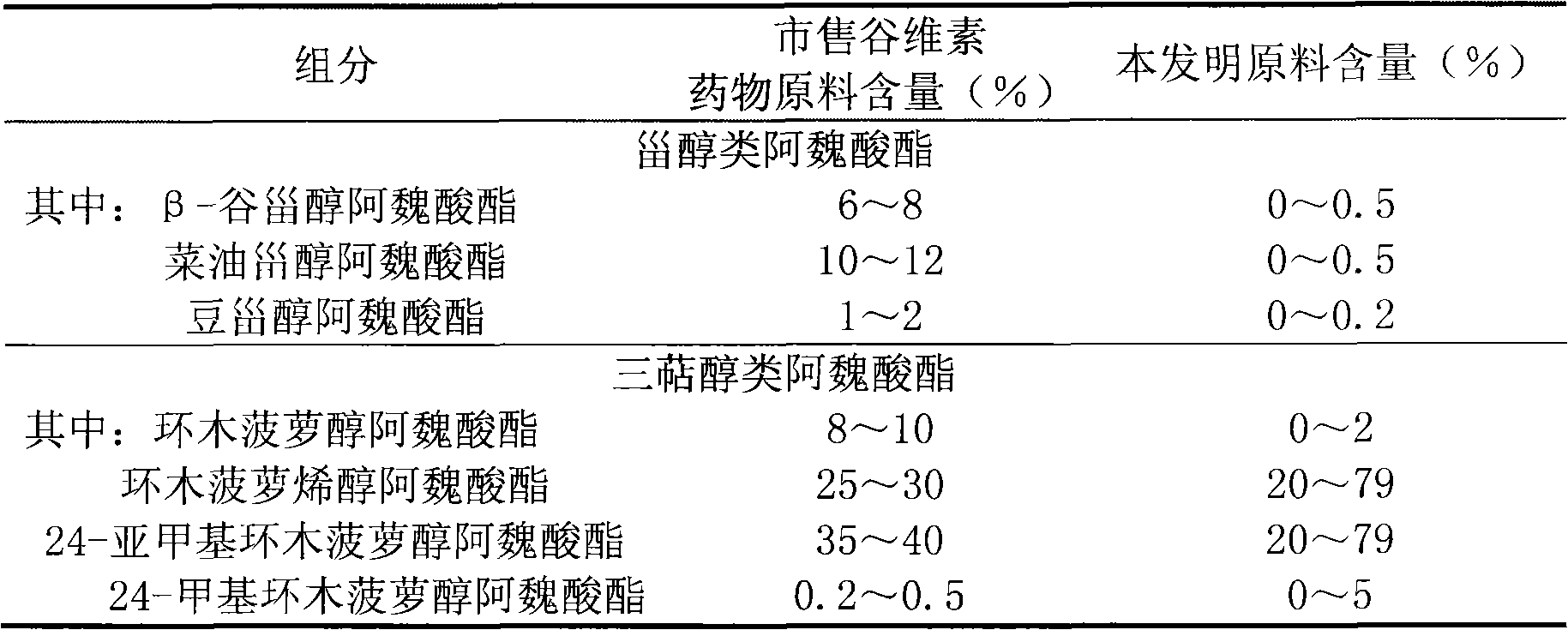
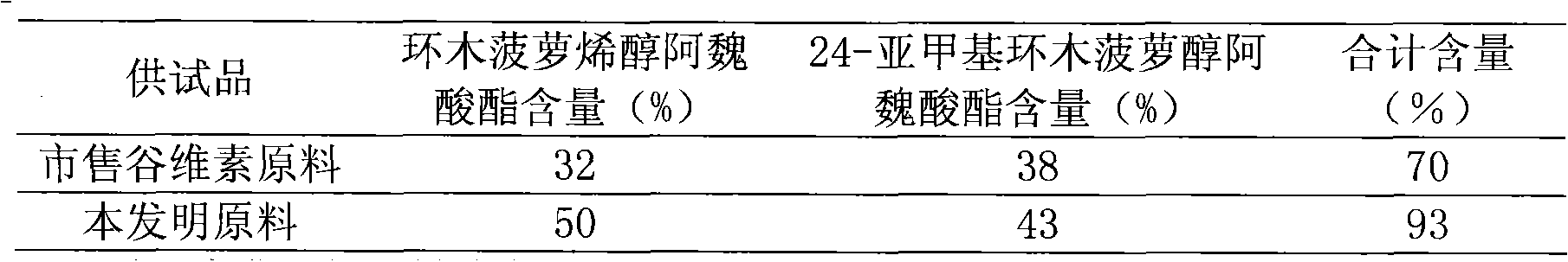
[0053] 4. Measurement results
[0054] Table 2 Measurement results
[0055]
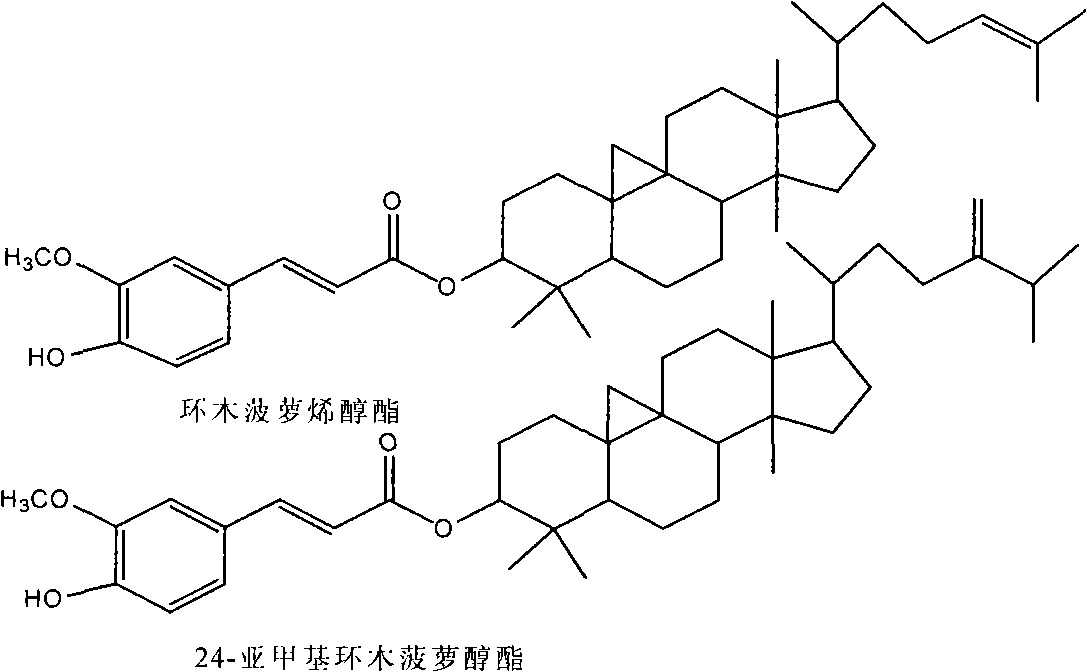
[0056] Illustrate that the raw materi...
Embodiment 3
[0058] prescription
[0059] 1000 bottles of freeze-dried powder of the present invention for injection (containing 20 mg / bottle of the present invention):
[0060] Raw material 20g of the present invention
[0061] Macrogol 400 250g
[0062] Tween 80 250g
[0063] 10% Mannitol 6000g
[0064] Add water for injection to 5000ml
[0065]
[0066] A total of 1000 tubes were freeze-dried
[0067] In the prescription, the raw material of the present invention is used as the main agent, Tween 80 is used as a solubilizing agent, polyethylene glycol 400 is used as a cosolvent, mannitol is used as a proppant, and water for injection is used as a freeze-drying solvent.
[0068] Preparation process
[0069] Weigh the raw material of the present invention, Tween-80, PEG-400, mannitol and 80% of the prescribed amount of water for injection, stir to make the raw material of the present invention dissolve until the solution is clear, stir at room temperature...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com