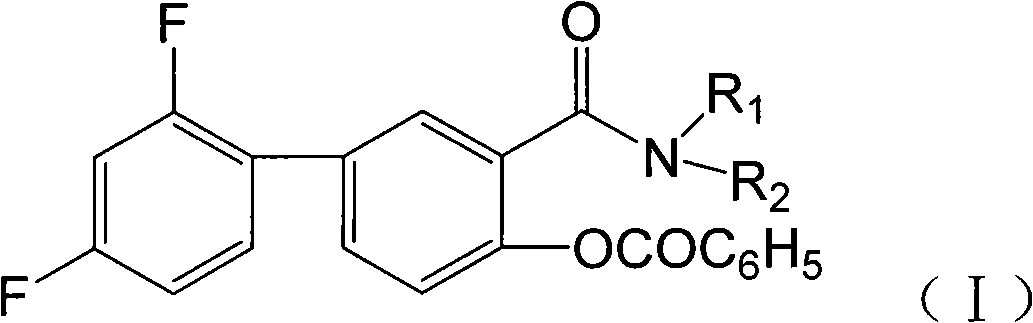
Benzoyl fluoride benzene salicylamide compounds, preparation and application thereof
A technology of benzoyl phenylsalicylamide and benzoyl fluoride, which is applied in the field of preparation of anti-inflammatory and analgesic drugs, and can solve diflunisal poor solubility, side effects, and application limitations and other problems, to achieve the effect of easy industrial production, significant analgesic effect, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: Preparation of benzoyl fluorophenyl salicylic acid (II)
[0037]
[0038] Add 15g (0.06mol) of diflunisal, 50ml of dichloromethane, and 4.8g (0.06mol) of pyridine in sequence in the reaction flask, stir, add dropwise 8.5g (0.06mol) of benzoyl chloride, stir, and react at room temperature for 4h. After the reaction is completed, wash with dilute hydrochloric acid, suction filter, and dry to obtain 20.1 g of white solid, which is the crude product of benzoylfluorophenylsalicylic acid (II) (purity ≥ 85%), melting point: 167-172°C, and set aside;
[0039] 1 H NMR (500MHz, CDCl 3 ): δ (ppm): 6.96 (t, 1H, J = 8.0Hz, 3'-H), 7.00 (t, 1H, J = 8.0Hz, 5'-H), 7.35 (d, 1H, J = 7.5 Hz, 5-H), 7.46(m, 1H, 6'-H), 7.50(t, 2H, J=8.0Hz, 3", 5"-H), 7.65(t, 1H, J=7.6Hz, 4″-H), 7.80(d, 1H, J=8.0Hz, 6-H), 8.21(d, 2H, J=7.5Hz, 2″, 6″-H), 8.25(s, 1H, 2- H), 11.24(s, 1H, -COOH);
[0040] EIMS: m / z(%)=354(M + , 1.02).
Embodiment 2
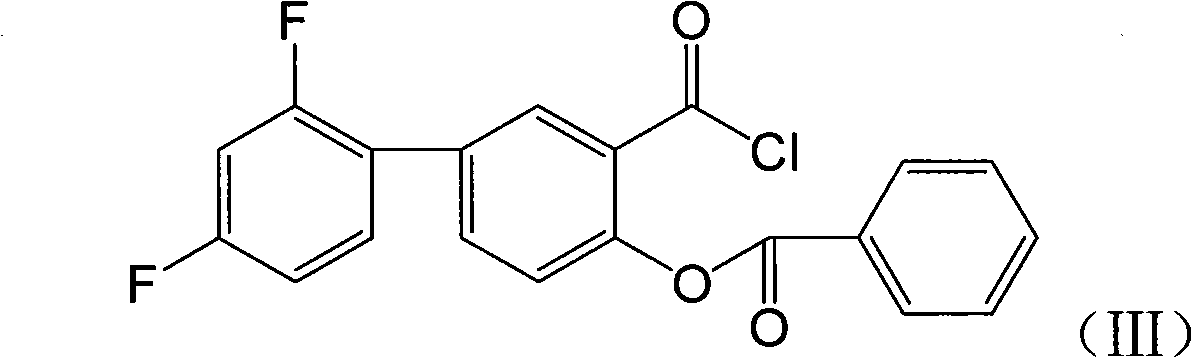
[0041] Embodiment 2: Preparation of benzoyl fluoride benzene salicyloyl chloride (III)
[0042]
[0043] The benzoyl fluorophenylsalicylic acid crude product 3.54g (0.01mol), sulfur oxychloride 1.8g and 30m1CH prepared in Example 1 2 Cl 2 Added to the reaction bottle, reflux reaction for 4 hours. After the reaction was completed, evaporate to dryness under reduced pressure to obtain a light yellow solid, which is the crude product of benzoyl fluoride phenylsalicyloyl chloride (III) (purity ≥ 80%), and set aside.
Embodiment 3
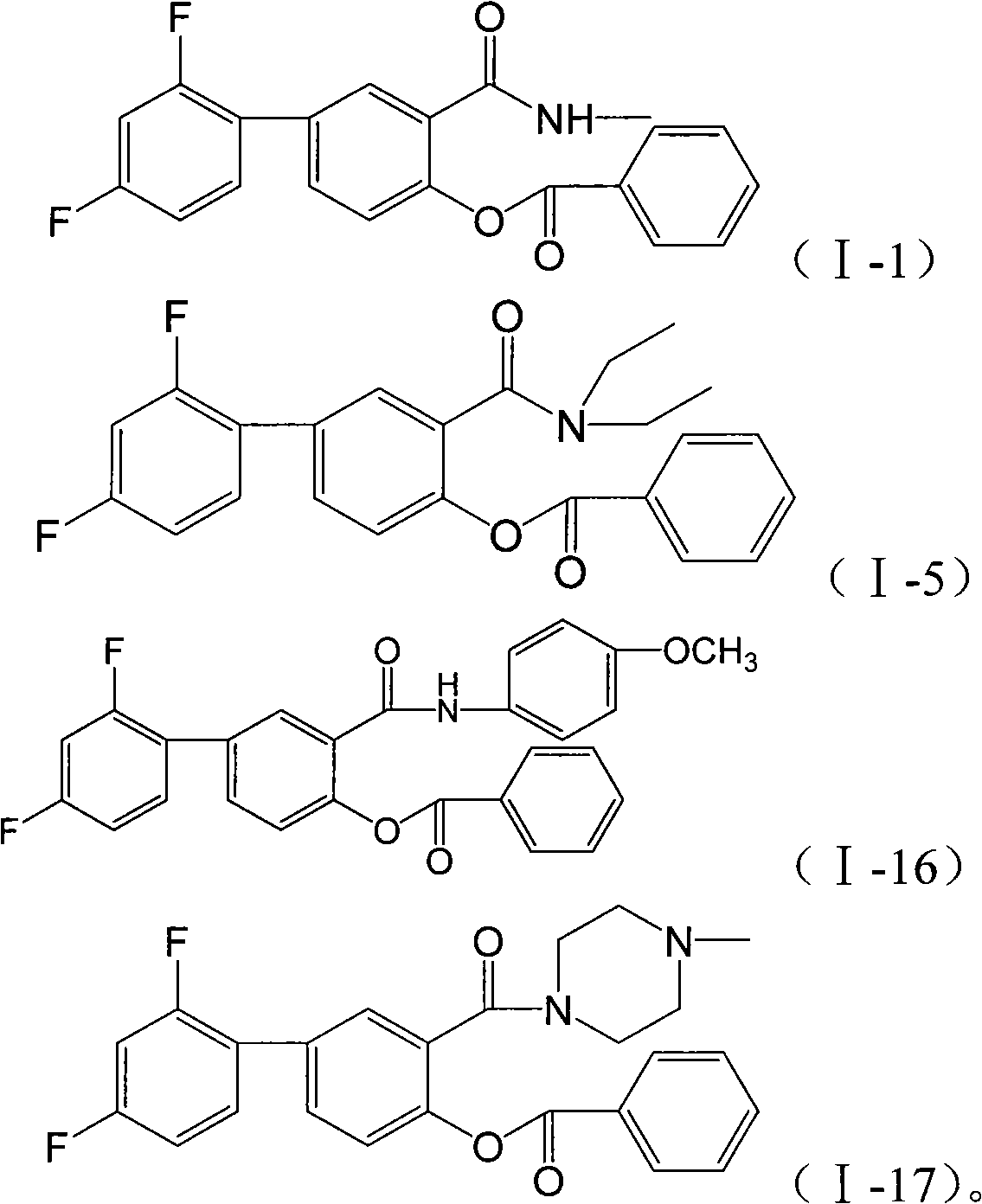
[0044] Embodiment 3: Preparation of N-methylbenzoyl fluorophenyl salicylamide (I-1)
[0045]
[0046] Add all the crude benzoyl fluoride benzene salicyloyl chloride and 30ml of dichloromethane into the reaction flask, stir, add dropwise 2.6g of 30% methylamine aqueous solution, and react at room temperature for 4h. Evaporate to dryness under reduced pressure and recrystallize to obtain 2.9 g of N-methylbenzoylfluorophenylsalicylamide, melting point: 147-150°C (uncorrected);
[0047] 1 H NMR (400MHz, CDCl 3 ): δ (ppm): 2.89 (d, 3H, J=4.8Hz, CH 3 ), 6.36(s, 1H, NH), 6.94(t, 1H, J=8.4Hz, 3′-H), 7.00(t, 1H, J=8.2Hz, 5′-H), 7.31(d, 1H , J=8.4Hz, 5-H), 7.45(m, 1H, 6′-H), 7.56(t, 2H, J=7.6Hz, 3″, 5″-H), 7.65(d, 1H, J =8.4Hz, 6-H), 7.69(t, 1H, J=7.6Hz, 4″-H), 7.94(s, 1H, 2-H), 8.27(d, 2H, J=6.8Hz, 2″ , 6″-H);
[0048] EIMS: m / z(%)=367(M + , 1.36).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com