Preparation of epothilones B lactam derivates
A technology of epothilone and lactam, which is applied in the field of producing epothilone B lactam derivatives, can solve the problems of lack of epothilone D, etc., and achieve the effect of high yield and short synthetic route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
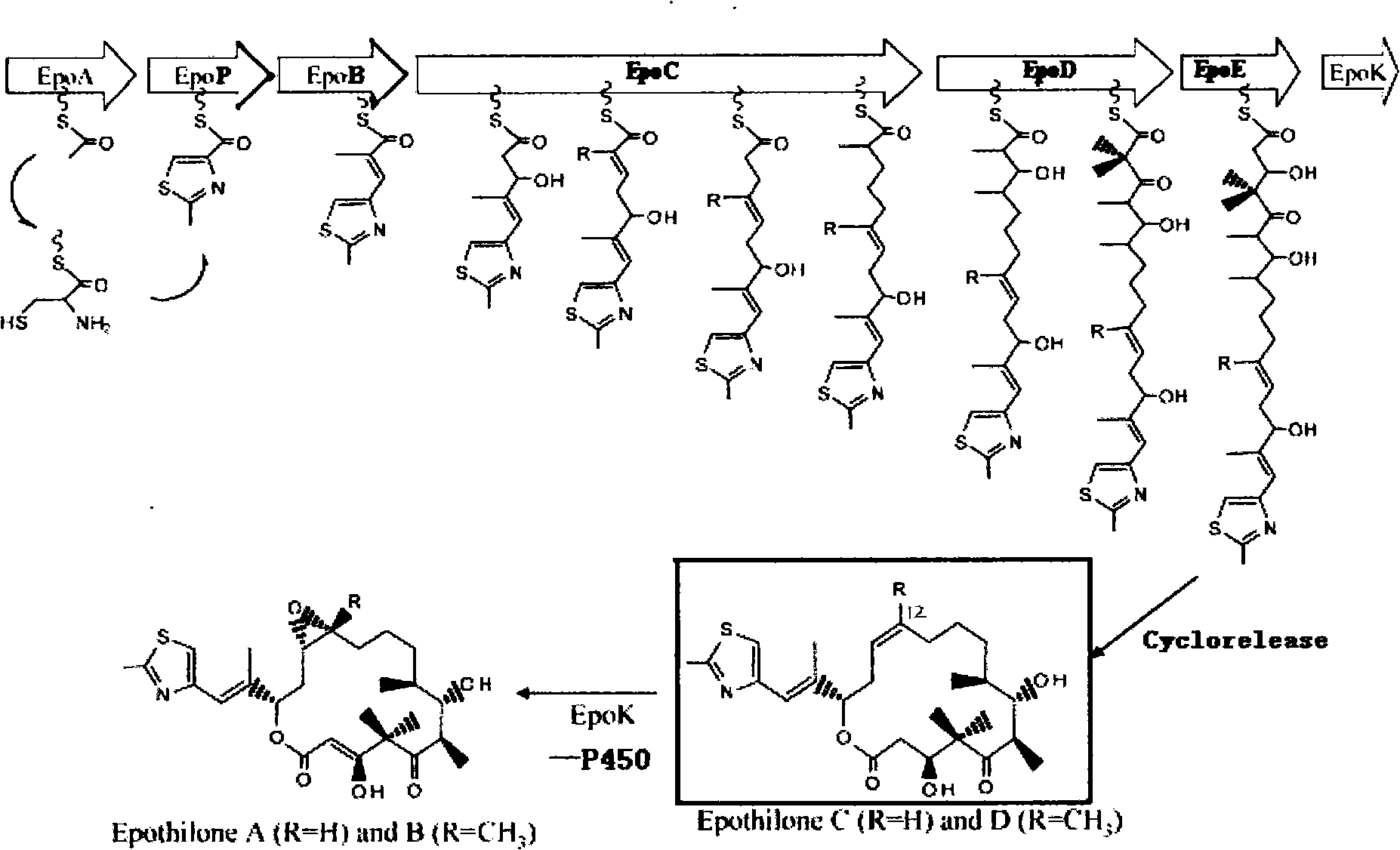
[0035] Gene mutation and directional screening to obtain a mutant strain of S. cellulosus containing the inactivated epoK gene
[0036] The starting strain was Sorangium cellulosum ATCC15384 among the myxobacteria strains, which was obtained from the American Type Culture Collection.
[0037] The above strains were inoculated on a solid medium, and the components of the solid medium on the slant were: soybean peptone 8g / L, potato starch 20g / L, yeast extract 10g / L, anhydrous glucose 5g / L, CaCl 2 ·H 2 O 1g / L, MgSO 4 ·7H 2 O 1g / L, Fe-EDTA 8mg / L, agar powder 15g / L. After culturing at 30°C for 5 days, it was inoculated into the liquid fermentation medium. The composition of the liquid fermentation medium is: soybean peptone 10g / L, potato starch 20g / L, yeast extract 4g / L, anhydrous glucose 15g / L, CaCl 2 ·H 2 O 1g / L, MgSO 4 ·7H 2 O 1g / L, Fe-EDTA 8mg / L, resin XAD-1620ml / L. 30°C, 120 rpm, shaker for 4 days. The epoK inhibitor Mytyrapone was added to a final concentration of 5...
Embodiment 2
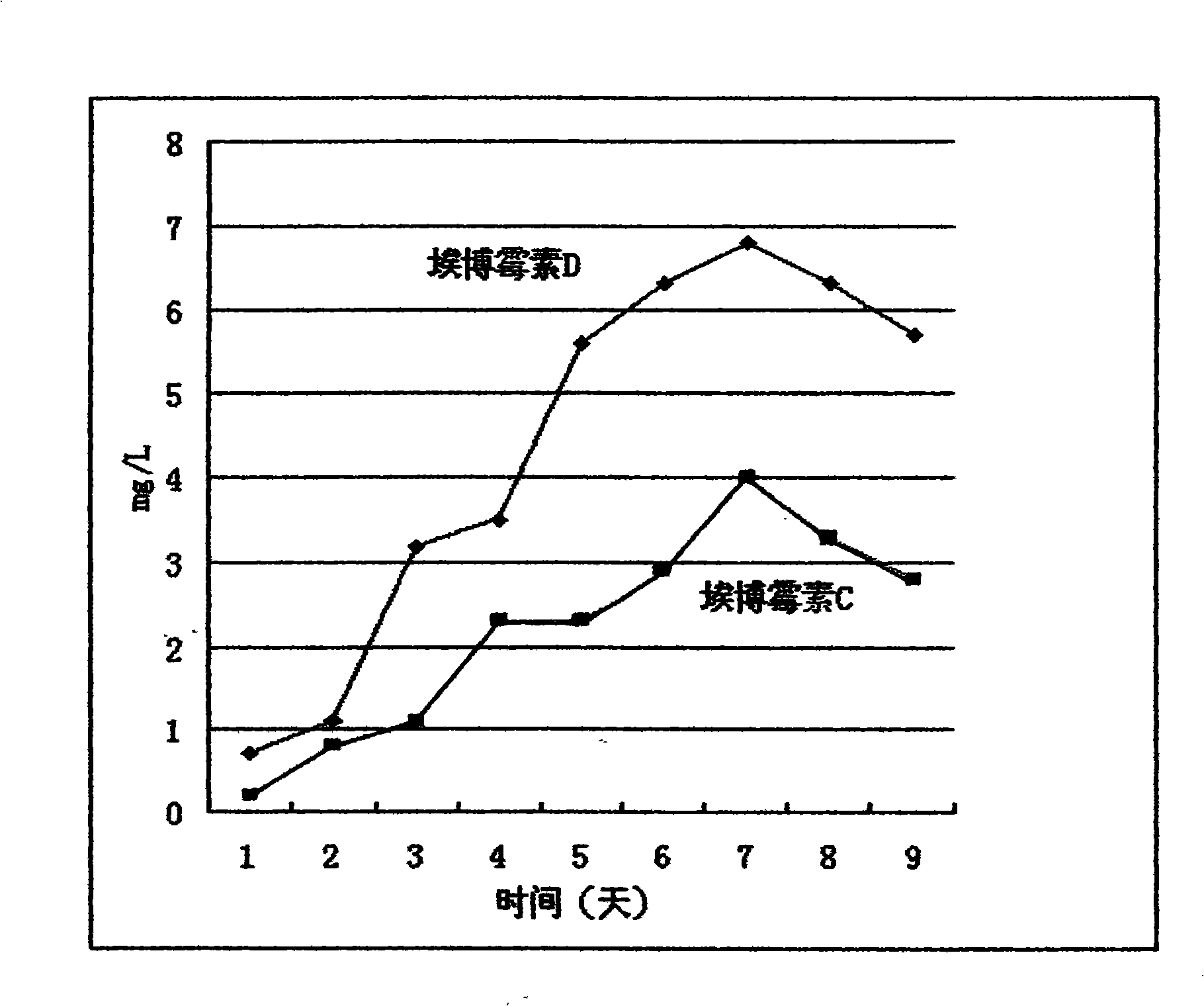
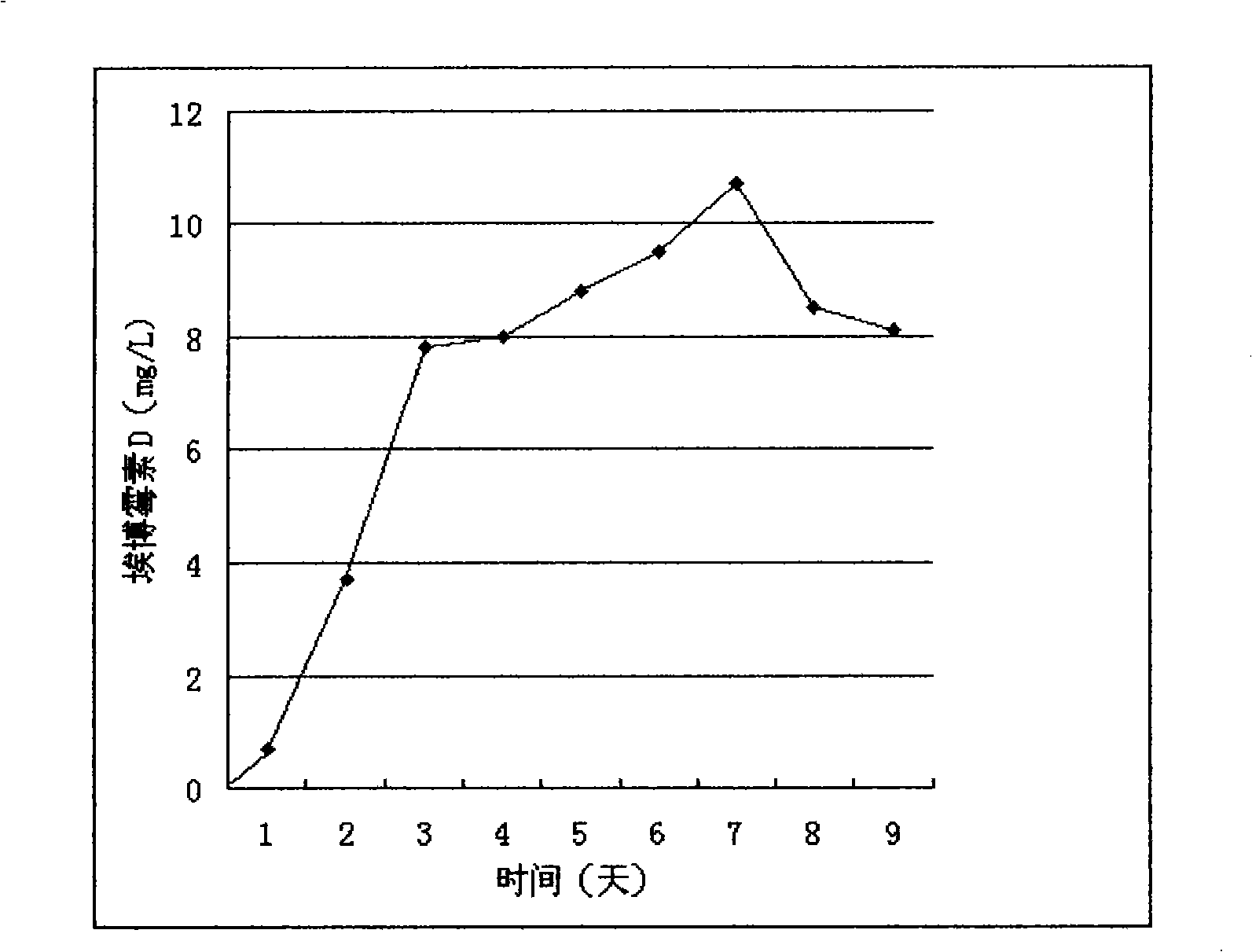
[0043] Fermentative production of epothilone D by mutant high-yielding strain
[0044] Continuous seed culture: 1ml of the frozen mutant high-yielding strain taken out of liquid nitrogen was inoculated into 10ml of G52 medium, and cultured at 30°C, 180 rpm, and 25mm displacement for 3 days. Add 5ml of seed culture to 45ml of G52 medium and grow for 3d. This 50ml culture was then added to 450ml G52 to grow for 3d. Every 3-4 days thereafter, 50 ml of the seed culture was inoculated into 450 ml of the G52 medium with a 10% inoculation amount to carry out continuous seed culture.
[0045] 20L intermediate seed culture: 18L G52 medium in a 30L fermenter, 2L of seed culture solution before inoculation, 30°C, 250 rpm, 0.5L air / L / min, and overpressure of 0.5bars for 3 days and 3 days ~4d, add 10ml of silicone antifoaming agent to prevent foam formation.
[0046] 250L fermentation culture:
[0047] 1B12 medium (potato starch Noredux A-150 (Blattmann, Waedenswil, Swizerland) 2%, def...
Embodiment 3
[0051] Synthesis of Epothilone B Lactam Derivatives Using Epothilone D as Starting Material
[0052] Epothilone D 129g (262.2mmol) was dissolved in 10.5L of anhydrous chloroform, m-CPBA 97.5g (390mmol) was added at -18°C, the reaction mixture was stirred at this temperature for 5h, and then diluted with 42L of dichloromethane, Add 45 L of saturated sodium bicarbonate to terminate the reaction. The layers were separated, the aqueous layer was extracted 3 times with dichloromethane, the combined organic phase was dried over magnesium sulfate, filtered and concentrated under reduced pressure, the residue was subjected to dynamic axial compression chromatography (silica gel, with n-hexane-ethyl acetate, 1:1 elution), to obtain epothilone B 108g (81%).
[0053] Epothilone B 101.2g (199.4mmol) and sodium azide 15.54g (240mmol) were suspended in THF-H 2 O (5:1) mixed solution 1.92L, degassed with nitrogen for 20min, then treated with a catalytic amount of triphenylphosphine palladi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com