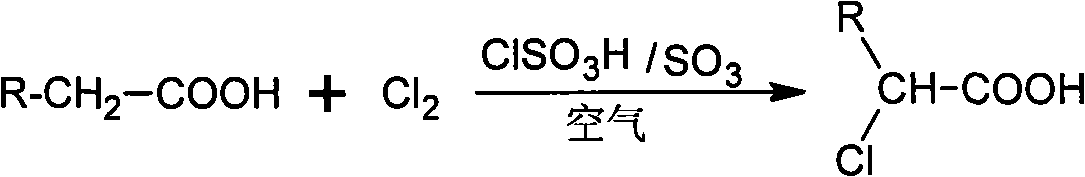
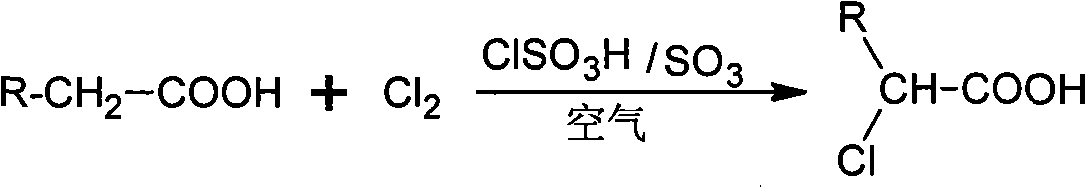
Method for preparing alpha-chloro-fatty acid
A technology for chlorinated fatty acids and fatty acids, applied in the field of organic compound synthesis, to achieve the effects of high activity, mild reaction conditions, and reduction of unsafe factors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] The synthesis of embodiment 1α-chlorohexanoic acid
[0019] Put 30g (0.26mol) of dry hexanoic acid into a high aspect ratio reaction kettle with acid-resistant gas distributor, mechanical stirring and jacket, heat it to melt and raise the temperature to 70°C, add 1.0g of chlorosulfur dropwise Acid, feed the mixed gas of chlorine gas and air (the volume flow ratio of chlorine gas and air is 1:1), the amount of chlorine gas is about 0.4mol, after reacting for 1h, add 0.5g sulfur trioxide continuously, and then stir at the reaction temperature for 3h After the reaction, the residual chlorine gas was driven away with nitrogen, and the temperature was lowered to 50° C. for discharge; the catalyst was separated and removed by a centrifuge to obtain 40 g of crude product of α-chlorohexanoic acid.
[0020] The content analysis method is as follows:
[0021] Gas chromatography (GC) conditions: PEG-20000 capillary column (30m × 0.32mm); FID detector; column temperature 200°C; in...
Embodiment 2
[0024] Synthesis of embodiment 2α-chlorododecanoic acid
[0025] Put 100g (0.5mol) of dry dodecanoic acid into a high aspect ratio reaction kettle with acid corrosion gas distributor, mechanical stirring and jacket, heat it to melt and raise the temperature to 120°C, add 1.0g of chlorine dropwise Sulfonic acid, feed the mixed gas of chlorine gas and air (the volumetric flow ratio of chlorine gas and air is 2:1), the amount of chlorine gas is about 1.5mol, after reacting for 0.5h, add 1.0g sulfur trioxide continuously, and then keep the temperature at the reaction temperature After 3 hours, the residual chlorine gas was driven away with nitrogen gas after the reaction was completed, the temperature was lowered to 50° C., and the material was discharged; the catalyst was removed by high-speed centrifugation in a centrifuge to obtain 119 g of crude product of α-chlorododecanoic acid. The content analysis method is the same as in Example 1, and the yield of α-chlorododecanoic acid...
Embodiment 3
[0026] The synthesis of embodiment 3α-chlorohexadecanoic acid
[0027] Put 500g (1.95mol) of dry hexadecanic acid into a high aspect ratio reactor with an acid-resistant gas distributor, mechanical stirring and jacket, heat it to melt and raise the temperature to 100°C, and add 3.0g of Chlorosulfonic acid, pass into the mixed gas of chlorine and air (the volumetric flow ratio of chlorine and air is 5:1), the amount of chlorine is about 2.5mol, after reacting for 1.5h, add 2.0g sulfur trioxide continuously, then under the reaction temperature Stir at constant temperature for 4 hours. After the reaction, use nitrogen to drive away residual chlorine gas, cool down to 50°C and discharge; use a centrifuge to separate and remove the catalyst to obtain 570g of crude product of α-chlorohexadecanoic acid. The content analysis method is the same as in Example 1, and the yield of α-chlorohexadecanoic acid is 97.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com