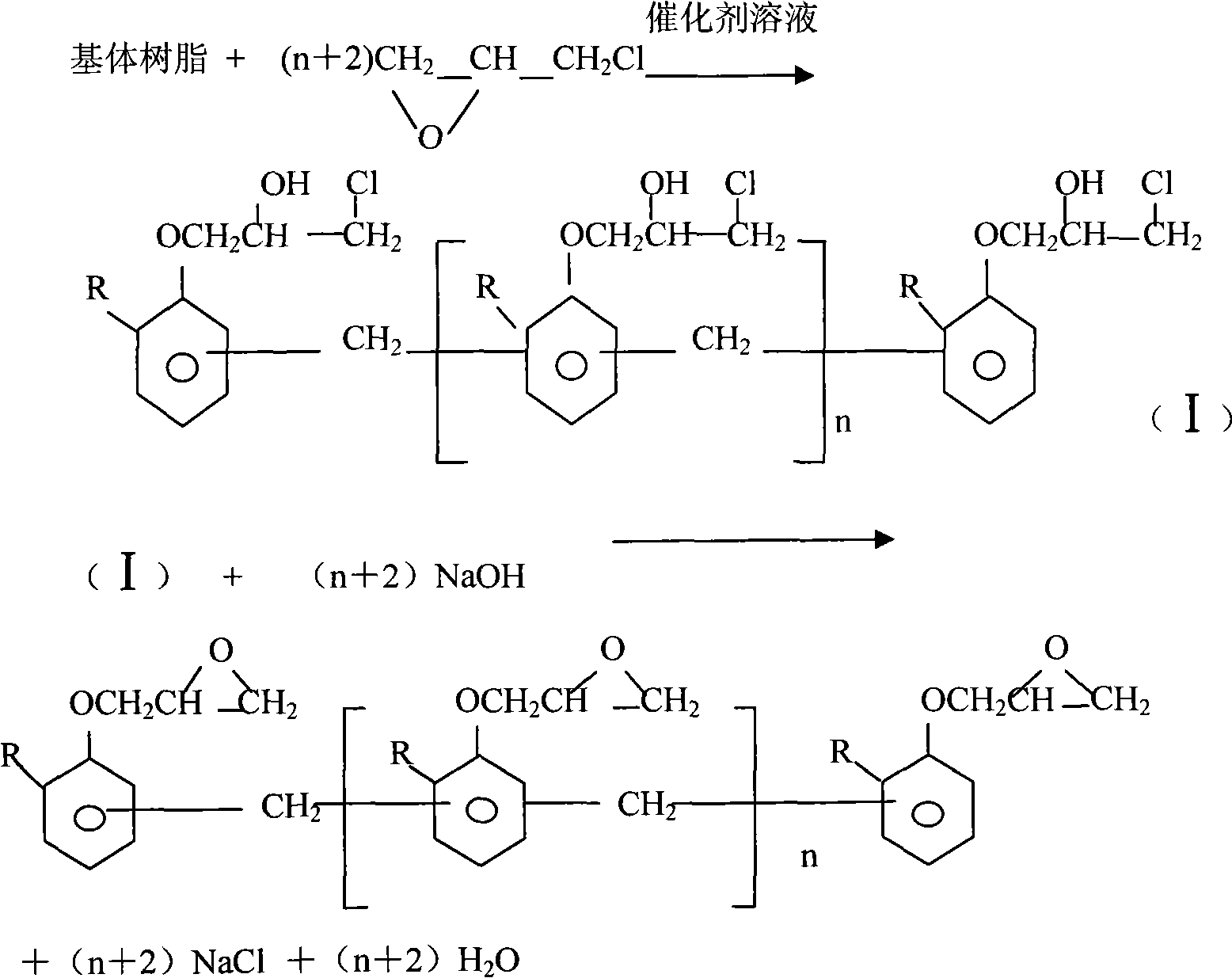
Method for preparing epoxy resins of phenol formaldehyde type
A technology of epoxy resin and phenol formaldehyde, which is applied in the field of epoxidation, can solve the problems of insufficient etherification reaction, increase of resin chroma, and many side reactions of hydrolysis, so as to reduce hydrolysis side reactions, accelerate etherification reaction, reduce Occurrence Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032]Be that 105 ℃ of linear phenol formaldehyde resin 1000g with softening point and 6000g purity are 99.5% (weight) epichlorohydrin drop into reaction container, make system material be in purity (weight) be under the nitrogen protection of 99.5%, temperature control is at Dissolve at 80-82°C for 50 minutes to form a uniform transparent liquid, then control the reaction system at a vacuum of 0.082-0.083MPa, a temperature of 75±2°C, and an epichlorohydrin reflux rate of 1100-1200g / h, and add all at once 10g of benzyltriethylammonium chloride aqueous solution with a weight concentration of 50%, and keep it for 15 minutes, then add 820g of NaOH aqueous solution with a weight concentration of 48% to the system dropwise, and adjust and control the reflux rate at about 1100-1200g / h during the dropping process Carry out water separation and cyclization reaction, add sodium hydroxide solution evenly for 4 hours, maintain the etherification reaction for 2 hours under the same conditi...
Embodiment 2
[0036] Be that 1000g of matrix resin linear phenol-formaldehyde resin 1000g and 5000g purity are 99.5% (weight) epichlorohydrin drop into reaction container with softening point, make system material be in purity (weight) under the nitrogen protection of 99.5%, control Dissolve at 78-80°C for 40 minutes to form a uniform transparent liquid, then control the reaction system at a vacuum of 0.082-0.083MPa, a temperature of 75±2°C, and a reflux rate of epichlorohydrin of 1100-1200g / h. Add 8g of tetrabutylammonium chloride and tetramethylammonium chloride mixture aqueous solution with a weight concentration of 50% and keep it for 12 minutes, then add 800g of KOH aqueous solution with a weight concentration of 50% to the system dropwise, and keep the vacuum during the dropping process At 0.078-0.080MPa, the reaction temperature is 72-73°C, the reflux rate is controlled at about 1100-1200g / h to carry out the water separation cyclization reaction, and the potassium hydroxide solution i...
Embodiment 3
[0040] Put 1000g of o-cresol formaldehyde resin with a softening point of 100°C and 6000g of epichlorohydrin with a purity of 99.5% into the reaction vessel, so that the system material is under the protection of nitrogen with a purity (weight) of 99.5%, and the temperature is controlled at 80-100%. Dissolve at 82°C for 60 minutes to form a uniform transparent liquid, then control the reaction system at a vacuum of 0.078-0.082MPa, a temperature of 75±2°C, and a reflux rate of epichlorohydrin of 1200-1300g / h, and add the weight all at once Concentration 50% benzyltriethylammonium bromide and the dipropylene glycol solution 10g of triphenylethylammonium iodide mixture, after keeping 20 minutes, in the system, drip the NaOH aqueous solution 850g of weight concentration 49% again, dropwise During the process, keep the vacuum degree at 0.078-0.082MPa, the reaction temperature at 75±2°C, adjust and control the reflux flow at about 1200-1300g / h to carry out the water separation and cy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| softening point | aaaaa | aaaaa |
| epoxy equivalent | aaaaa | aaaaa |
| softening point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com