Method for separating efficacy ingredient from solid body and semi-solid formulation
A technology for functional components and semi-solids, applied in the field of separation of functional components particles, can solve the problems of application approval, detection and analysis, no way to detect, etc., and achieve the effect of system specification, high precision, and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The separation of functional components in embodiment 1 Nuoka cream, gel, ointment dosage form and suppository
[0035] Verification example of the present invention selects Nuoka emulsifiable cream dosage form, Nuoka gel dosage form, Nuoka ointment dosage form and Nuoka suppository dosage form containing trace functional components developed by Shenyang Shengbaokang Biopharmaceutical Co., Ltd., according to the method of the present invention, respectively The four dosage forms were subjected to the separation experiment of trace functional components.
[0036] 1) Separation of functional components in Nuoka cream
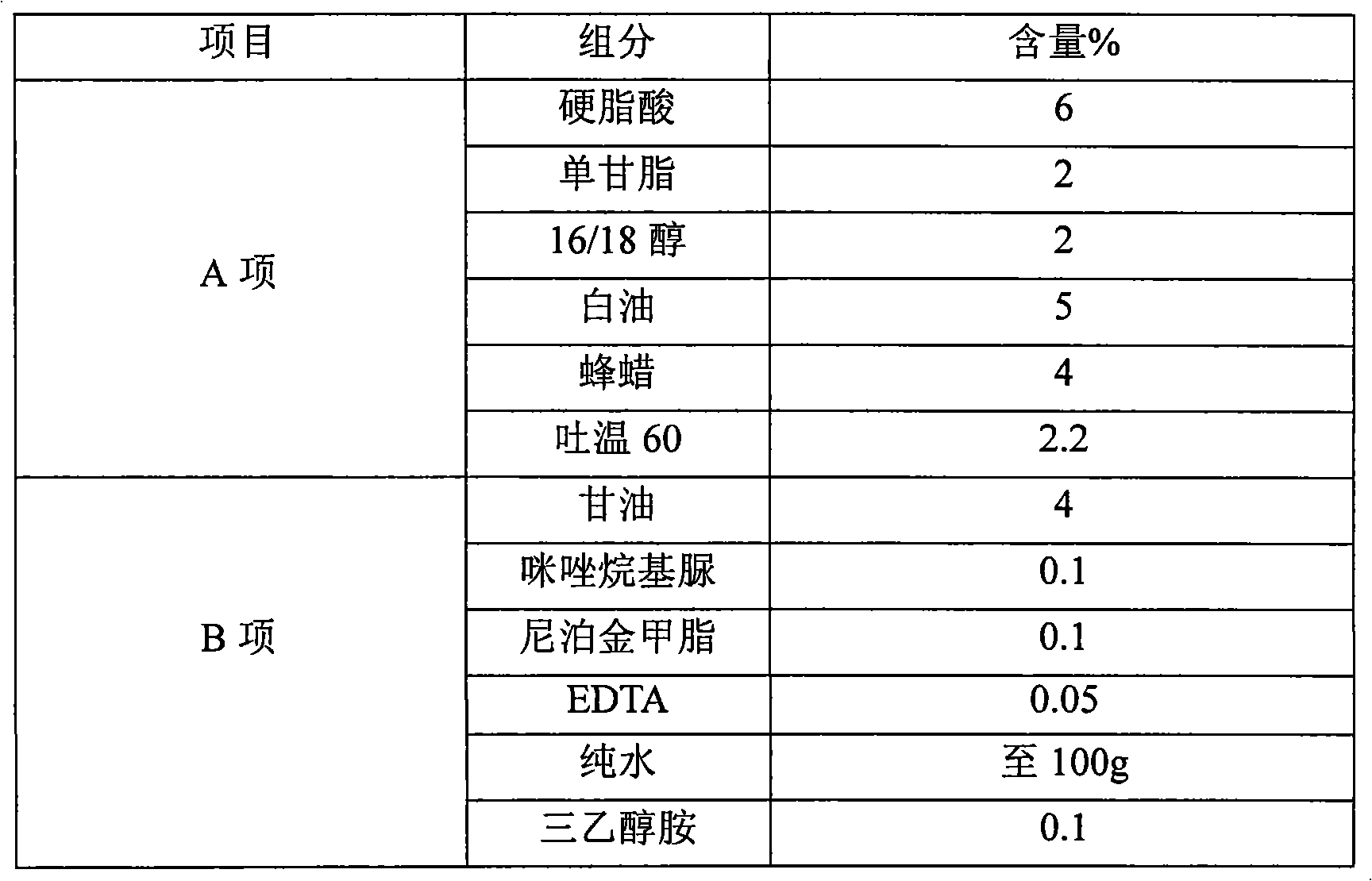
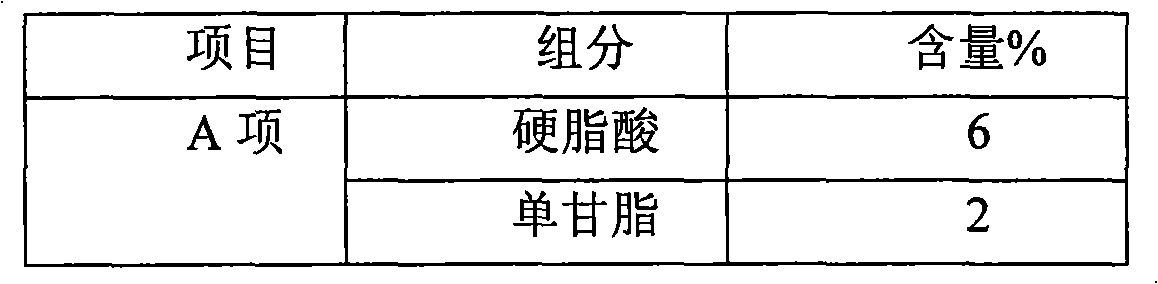
[0037] Among them, the Nuoka cream under inspection belongs to the oil-in-water cream base, and the commonly used emulsifier triethanolamine, 16 / 18 alcohol, monoglyceride, stearic acid and other raw materials are mainly used, which meet the requirements of the national pharmacopoeia.
[0038] The key functional component particles of the Nocardia cream are...
Embodiment 2
[0143] The separation of keel powder in embodiment 2 keel powder emulsifiable paste
[0144] The most common medicinal keels are the bones or teeth of horses, sheep, cattle, pigs, deer, elephants, rhinos, camels, etc., and carnivores, which are often off-white. Dragon bone is regarded as one of the important medicines in traditional Chinese medicine. Li Shizhen’s "Compendium of Materia Medica" "Dragon" discusses "long bone" and its indications: hemiplegia, semen and old charm. Intense knots, children are frightened by heat.
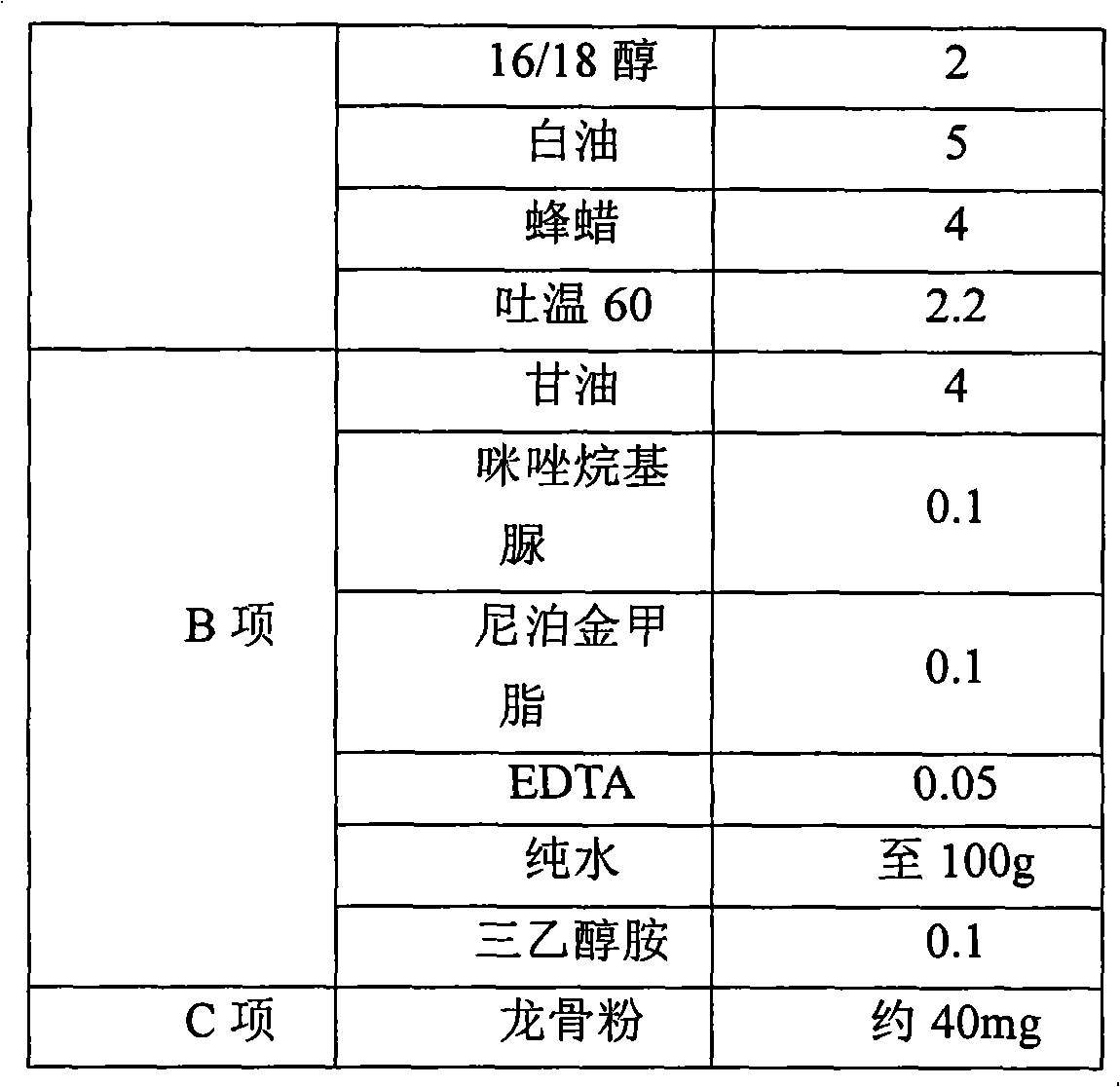
[0145] The content of keel powder per gram in homemade keel powder cream is ≥400 μg, the content is low, the particle size is less than 150 μm, and it exists in the cream system. In this experiment, keel powder in keel powder cream was separated by physical methods and its content was determined.
[0146] Experimental steps:
[0147] 1. Preparation of keel powder cream
[0148] First pass the keel powder through a 100-mesh sieve to obtain keel particle...
Embodiment 3
[0176] The separation of nano-silver in the nano-silver gel of embodiment 3
[0177] Nano silver gel has anti-inflammatory and bactericidal effects. The main component is nano-silver in the gel, its diameter is only 8-10nm, and the content in the gel is only a few hundred micrograms. Therefore, the qualitative and quantitative inspection of nano-silver in the nano-silver gel has brought many difficulties. This time The test is to separate the nano-silver through some physical methods, and to conduct qualitative and quantitative tests.
[0178] Sample: nano-silver antibacterial hydrogel, specification: 3ml / bottle, nano-silver content ≥ 350μg per ml gel, Changchun Kexin Institute of Chemical Medicine and Equipment.
[0179] Experimental steps:
[0180] 1. Dilute, take the nano-silver gel, dilute it into 4 samples in a 10ml centrifuge tube according to the table below
[0181]
Sample 1
Sample 2
Sample 3
Sample 4
blank
Nano silver content (μg...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com