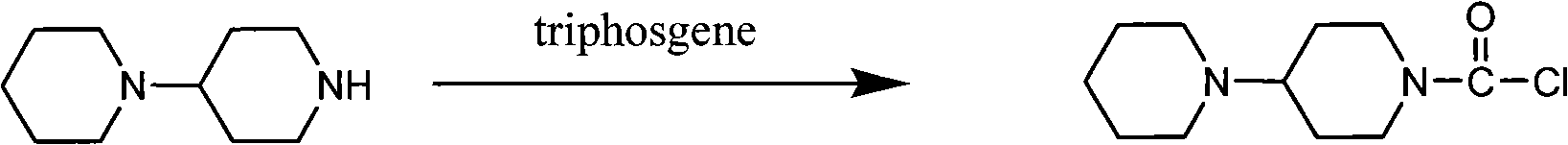Method for preparing high-purity irinotecan
An irinotecan, high-purity technology, applied in the fields of low temperature treatment, mixed solvent refining and impurity removal, raw material and intermediate purification, can solve the problems of poor water solubility and ester solubility, high price, etc., and achieves less solvent consumption and better effect. Good, the effect of reducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
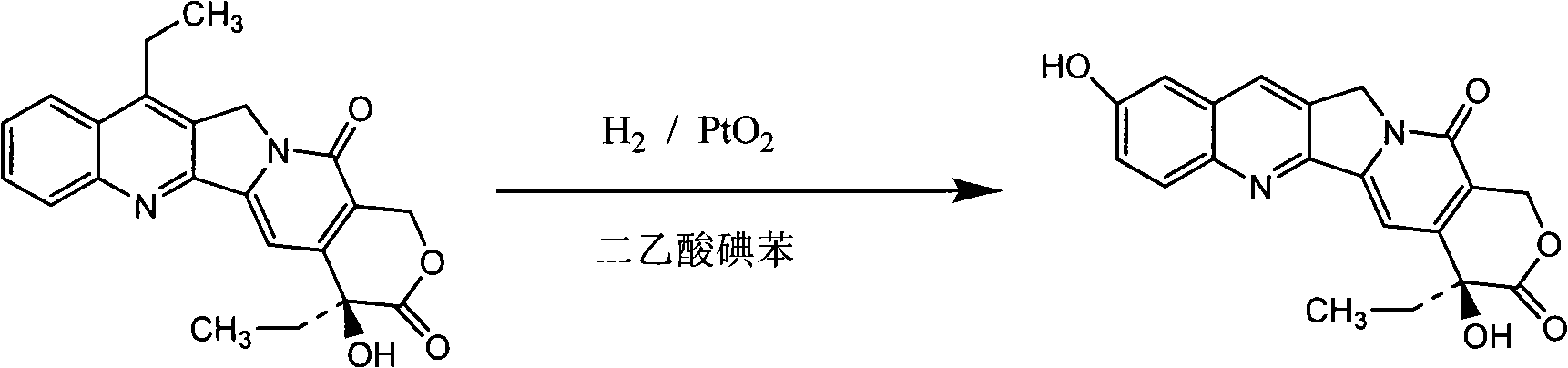
[0029] Example 1 Preparation of 7-Ethyl-10-Hydroxycamptothecin
[0030] Dissolve 38g of 7-ethylcamptothecin in 350ml of glacial acetic acid, add 215ml of DMSO, put it in a 1L autoclave, add PtO 2 6g, reacted at 70°C, 5bar, rotating speed 800rpm, cooled to 25°C after 12 hours, exchanged hydrogen with nitrogen, filtered, washed the filter cake with 20ml of glacial acetic acid, added 110ml of water to the resulting solution, 60g of iodobenzene diacetate, in 25 Stir at ℃ for 15 minutes, concentrate, add 700ml of acetonitrile, a large amount of solid precipitates, super crushed in ultrasonic wave until uniform, filtered, and dried to obtain 31.5g of brown powder.
[0031] Dissolve the above solid in 240ml of DMF, rinse in 1200ml of ethanol, cool to room temperature, filter and wash with ethanol to obtain 25.5g of a light yellow solid, reflux the solid with 255ml of chloroform and 25.5ml of methanol for half an hour, cool to room temperature, and filter , and obtained 23.8g afte...
Embodiment 2
[0032] Example 2 Preparation of 4-piperidinylpiperidinecarbonyl chloride
[0033] Add 20.4g of triphosgene and 140ml of dichloromethane into the reaction flask, stir until dissolved, and cool to 0-5°C in an ice-salt bath. A mixture containing 11 g of 4-piperidinylpiperidine, 28 ml of triethylamine, and 140 ml of dichloromethane was added dropwise, and the drop was completed in about 1 hour. The reaction temperature was controlled below 5°C and stirred for 6 hours. When concentrated to about half the volume, filter. The filter cake was washed with a little dichloromethane, the dichloromethane layer was concentrated to a volume of about 60ml, 60ml of toluene was added, a white solid was precipitated, filtered, washed with toluene, and dried to obtain 15.7g of 4-piperidinylpiperidinecarbonyl chloride hydrochloride.
Embodiment 3
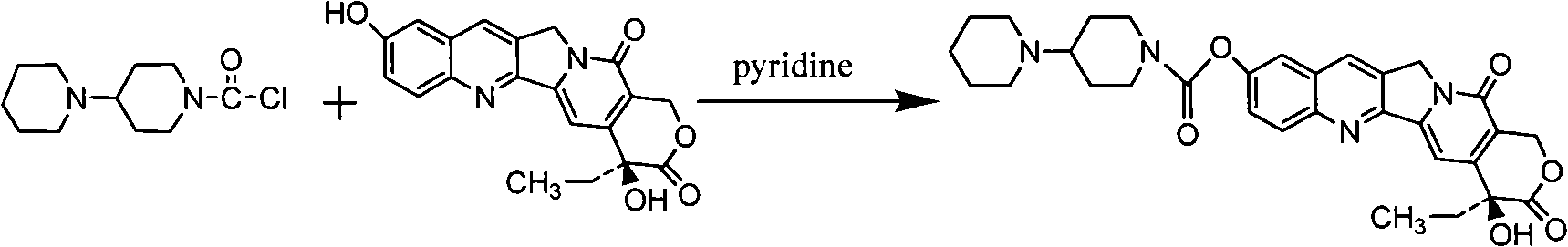
[0034] Example 3 Preparation of Crude Irinotecan
[0035] Put 15g of 7-ethyl-10-hydroxycamptothecin, 200ml of pyridine, and 100ml of dichloromethane into the reaction bottle, stir, and drop 15.7g of 4-piperidinylpiperidinecarbonyl chloride hydrochloride and di A solution of 50ml of methyl chloride was dripped for about half an hour. React at 20°C for 1 hour. Concentrate below 25°C for 10 minutes, rinse in 400ml of petroleum ether, filter, wash with petroleum ether, and dry to obtain a powdery solid.
[0036] Add 20ml of dichloromethane to dissolve the solid, adjust the pH to 1 with 4N hydrochloric acid ethanol, concentrate to dryness, add 60ml of water and 180ml of acetone, and stir and crystallize overnight. The next day, 17.5 g of pale yellow crystals were obtained by filtration.
[0037] Add 90ml of methanol and 18ml of water to 17.5g of the above solid, heat and dissolve, then concentrate under reduced pressure to obtain an oily substance, add 52.5ml of water, 157.5m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com