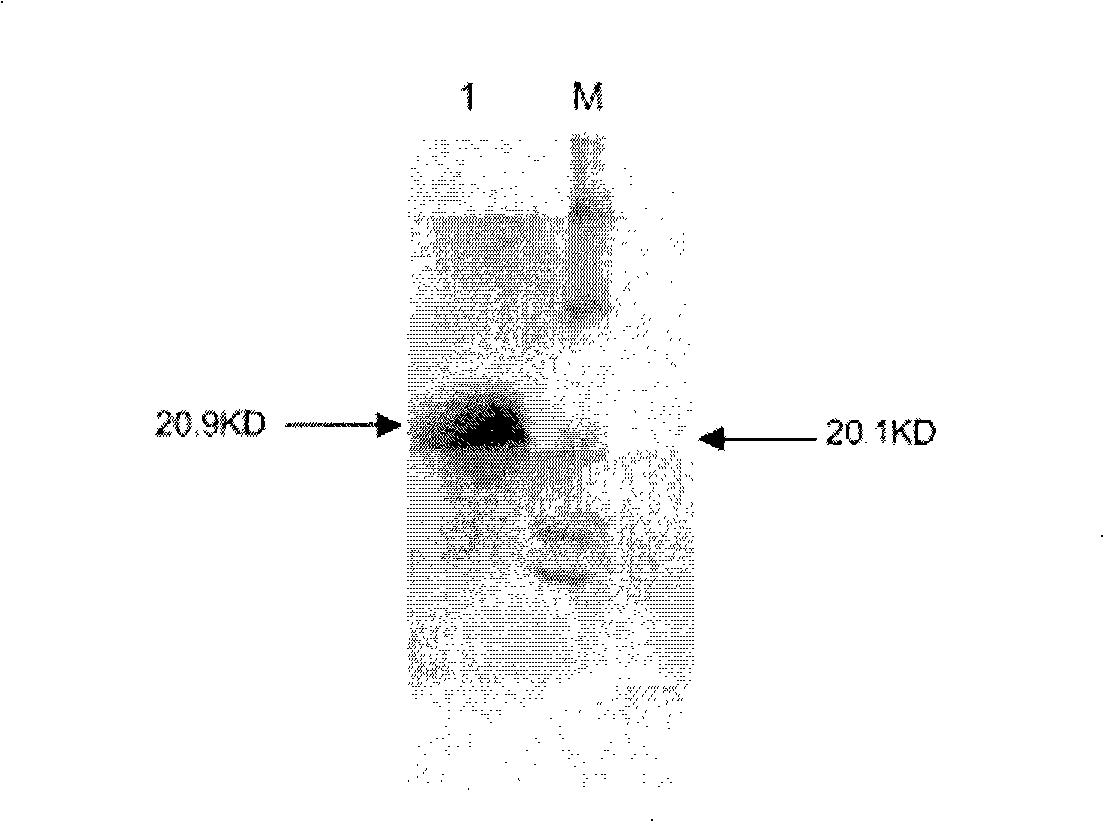Fusion protein of urokinase type plasminogen activator a chain and melittin and preparation thereof
A technology of fusion protein and plasminogen, which is applied in the direction of peptide/protein components, medical preparations containing active ingredients, hybrid peptides, etc., can solve the problem of loss of fibrin specificity and achieve inhibition of adhesion and efficient binding , The effect of targeting and killing tumor cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
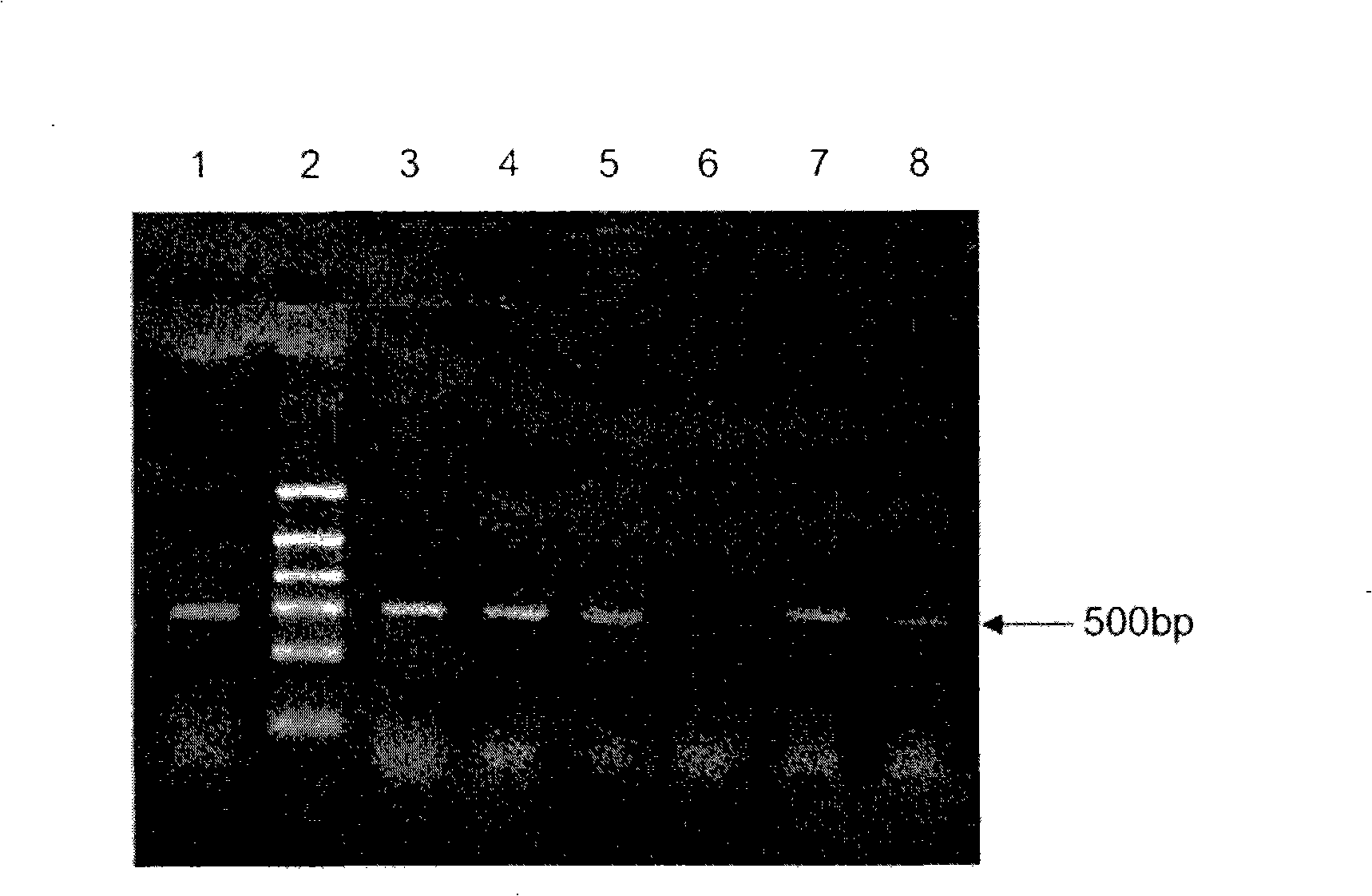
[0031] Example 1: Cloning and amplification of human uPA a chain gene
[0032] 1. Preparation of RNA:
[0033] Ovarian cancer tissues that were confirmed by rapid pathology as serous cystadenocarcinoma during the operation were put into liquid nitrogen for quick freezing. Using Trizol reagent (Invitrogen Corp. San Diego, California, USA.), ovarian cancer tissue RNA was extracted in one step. Specific steps are as follows:
[0034] Specifically, small pieces of frozen ovarian cancer tissue were first ground into a powder in a mortar filled with liquid nitrogen. Then, transfer the crushed tissue into a 1.5ml RNase-free EP tube, and add about 1ml Trizol. Add 200 μl of chloroform, shake vigorously, and place at room temperature for 30 seconds. After centrifugation (12 000r / min) at 4°C for 5 minutes, the supernatant was carefully transferred to another RNase-free EP tube. An equal volume of isopropanol was added thereto, left at room temperature for 5 minutes, centrifuged (12 ...
Embodiment 2
[0045] Example 2: Construction of huPAa-melittin Pichia secretory expression vector huPAa-melittin-pPICZα expression vector
[0046] 1. Construction of the huPAa gene eukaryotic expression vector backbone huPAa-X-pPICZα that can replace the uPAb chain at will
[0047] Take 100ng of the cloning plasmid that correctly contains huPAa identified by the above sequencing, and set up a PCR reaction system in a 0.2ml EP tube according to the following ratio:
[0048] huPAa recombinant plasmid 100ng; containing Mg 2+ 10μl of 10×LA PCR buffer; dNTPs (2.5mmol / L each) 8μl; upstream primer: 5'-CTG CTC GAG AAG AGA TCT AAC GAG CAC CAA GTTCCA TCG AAC-3'(SEQ ID NO: 3) 1μl ( 20pmol / μl); downstream primer: 5'-CAG GAATTC TCA AAT CTT AAA CCG CGG GCC TCA GAG TCT TTT-3' (SEQ ID NO: 4) 1μl (20pmol / μl); TaKaRa LA Taq (5U / μl) 1μl ; Sterilized ultrapure water to a final volume of 100 μl. Cyclic amplification was performed on a PCR machine. The amplification program was: 94°C for 4 minutes; 30 cycles...
Embodiment 3
[0060] Example 3: Establishment and screening of rhuPAa-melittin Pichia pastoris expression system
[0061] Take the culture medium with correct sequencing, extract the plasmid DNA according to the instructions of the plasmid extraction kit, and perform quantitative analysis by agarose gel electrophoresis. 20-25 μg of the expression vector huPAa-melitin-pPICZα was digested with SacI (linearized), extracted with phenol / chloroform and precipitated with ethanol. The linearized plasmid was dissolved in 10 μl of ultrapure water and kept on ice for later use.
[0062] Pick a single colony from the YPD negative culture plate of Pichia pastoris X-33 (YPD agar plate without antibiotics), inoculate it in 5ml YPD medium, culture at 250rpm, 30°C for 8 hours with shaking, and prepare yeast competent by conventional methods cell.
[0063] Then take 80 μl of the above-mentioned competent cells, mix them with 20-25 μg of linearized recombinant expression plasmids, transfer them into a 0.2 c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com