Pharmacy use of 4-aniline quinazoline derivatives
A compound and anti-tumor drug technology, applied in the application field of 4-aniline quinazoline derivatives in the preparation of anti-tumor drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
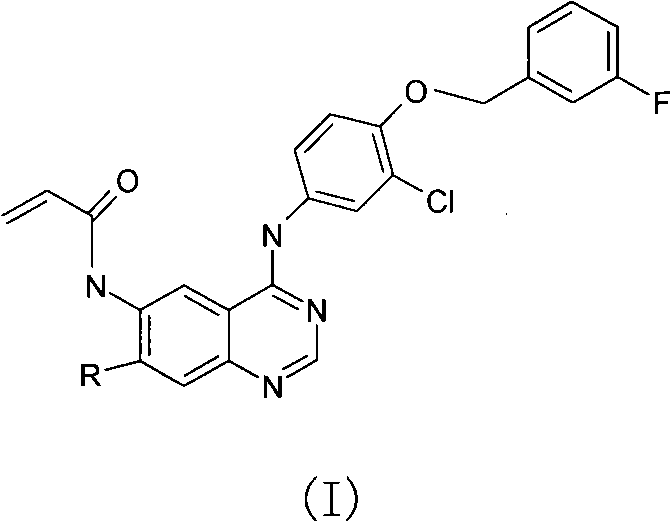
[0028] N-{4-[3-chloro-4-(3-fluoro-benzyloxy)phenylamino]-quinazolin-6-yl-}-acrylamide (Compound 1)
[0029]
[0030] In a flask equipped with a condensing device, dissolve 1.20 g (5.7 mmol) of raw material 6-nitro-4-chloro-quinazoline and 1.37 g (5.6 mmol) of 4-m-fluorobenzyloxy-3-chloroaniline In 80ml of isopropanol, reflux reaction for 3h, a large number of yellow solids precipitated in the system, filtered, and the solids were washed with saturated aqueous sodium bicarbonate until pH = 8. The sample was vacuum-dried, and the compound was identified as: 4-[3-chloro-4-(3-fluoro-benzyloxy)phenylamino]-6-nitroquinazoline, with a yield of 67%.
[0031]Add 4-[3-chloro-4-(3-fluoro-benzyloxy)phenylamino]-6-nitroquinazoline 1.60g (3.77mmol) in a flask equipped with a reflux condensing device, reduce Iron powder 1.05g (18.85mmol, 5eq), glacial acetic acid 2ml, methanol 40ml, reflux reaction in an oil bath at 85°C for 2.5h, remove iron powder by filtration, dilute the filtrate wit...
Embodiment 2
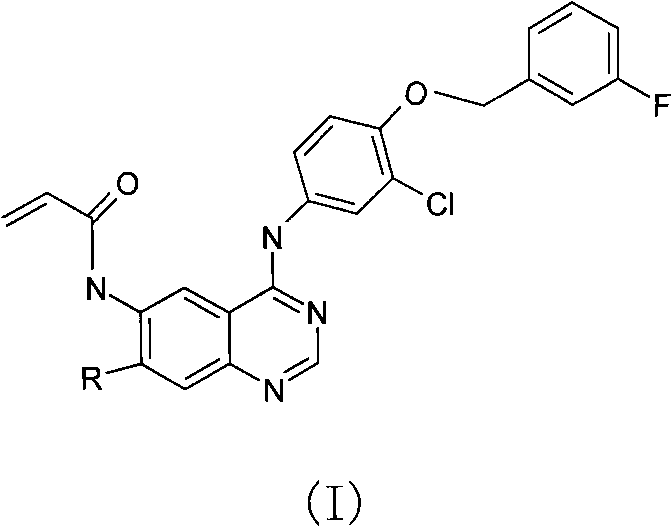
[0034] N-{4-[3-chloro-4-(3-fluoro-benzyloxy)phenylamino]-7-methoxy-quinazolin-6-yl-}-acrylamide (Compound 2)
[0035]
[0036] 10.0 g of 4-chloro-2-aminobenzoic acid was dissolved in 50 ml of formamide, refluxed for 5 hours, a large amount of solid precipitated, filtered and dried to obtain 11.5 g of 7-chloroquinazolone. Take 10.0g of quinazolon, slowly add it into 40ml of concentrated sulfuric acid and fuming nitric acid (1:1) mixed acid under ice bath, then raise the temperature to 90°C for 3h, the system becomes clear solution, carefully pour it into 300ml of ice water, and a pale yellow solid precipitates , filtered and washed with water, then dissolved in hot glacial acetic acid, 6-nitro-7-chloroquinazolone crystals were precipitated, and 6.50 g of the product was collected. Take 4.00g of the product and 15ml of phosphorus oxychloride to react with reflux for 2h, pour into ice water, filter and dry to obtain 6-nitro-4,7-dichloroquinazoline intermediate; dissolve it in ...
Embodiment 3
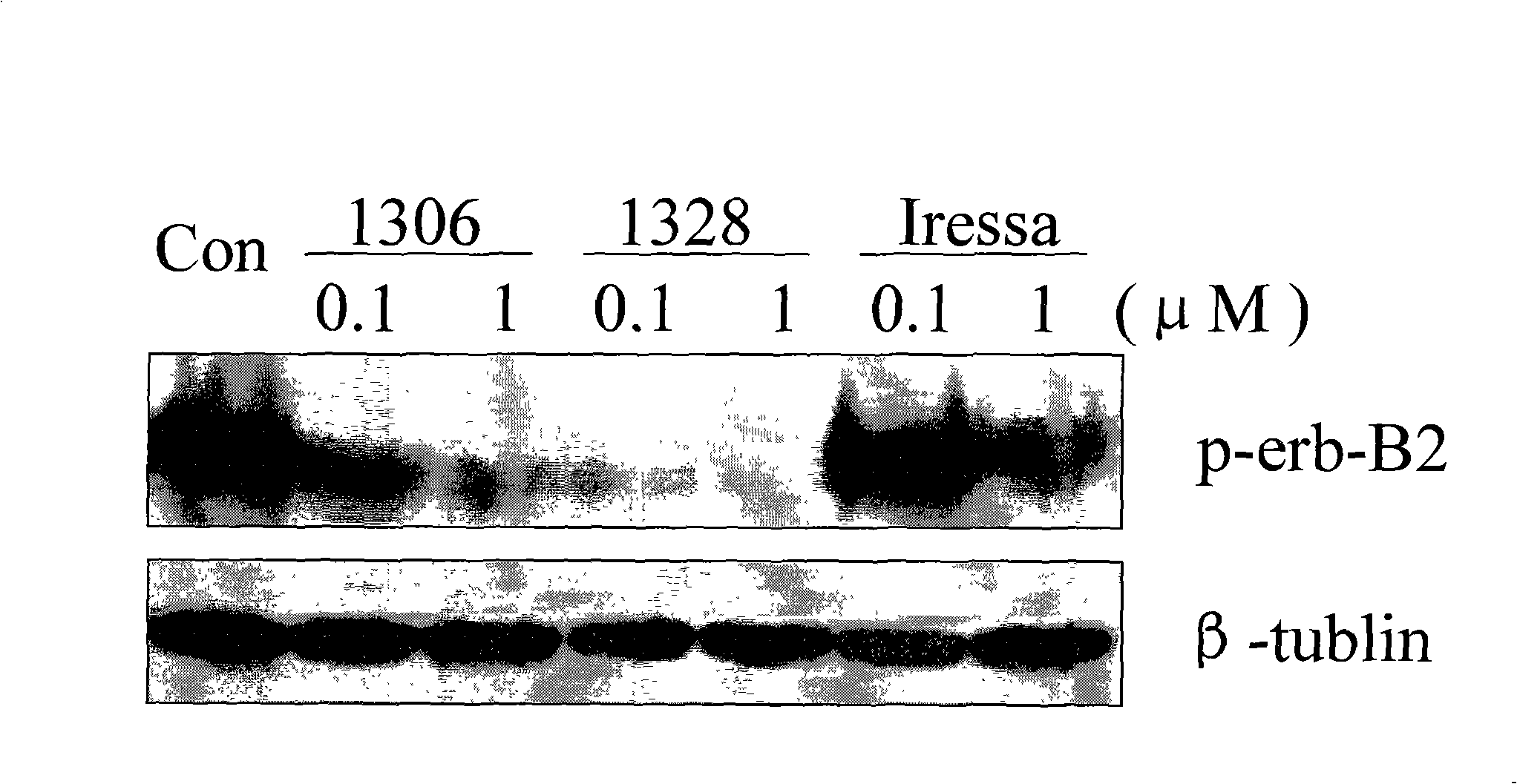
[0039] Embodiment 3: In vitro tumor cell inhibition experiment
[0040] Compounds 1 and 2 were formulated into 5 concentration gradients respectively, and 1×10 5 Different tumor cells, such as A431 (human epidermoid squamous cell carcinoma cells, high expression of erbB1 / low expression of erbB2), Calu-3 (human lung cancer cells, low expression of erbB1 / high expression of erbB2), BT-474 (human breast cancer cells, erbB1 low expression / erbB2 high expression), SKBR3 (human breast cancer cells, erbB1 low expression / erbB2 high expression), SKOV3 (human ovarian cancer cells, erbB1 low expression / erbB2 high expression) suspension 100ul inoculated in 96-well culture plate , and then add 10ul of different concentrations of medicinal solutions to reach the final concentration; place in a 37°C humid incubator, take out the culture plate after 72 hours, add MTT to each well, continue to cultivate for 6hrs, and add 100ul of SDS stop solution. Measure the optical density (OD) value of each...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com