Nickel-based catalyst
A nickel-based catalyst, alumina technology, applied in physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, etc., can solve the problems of low active specific surface and low nickel content, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
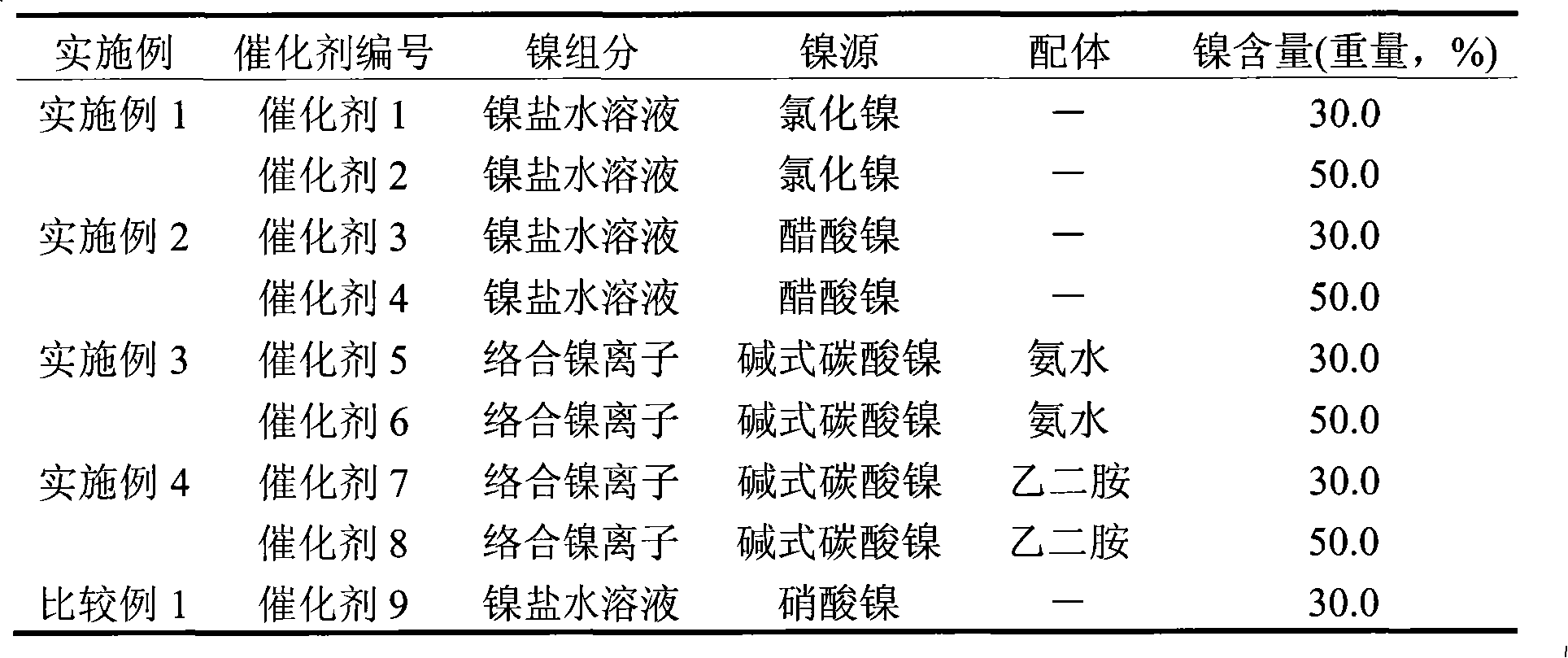
[0012] With nitric acid in [H + ] / [AlOOH] molar ratio of 0.25, the pseudo-boehmite powder was peptized for 24 hours to obtain an alumina sol with a solid content of 5% alumina. Nickel chloride was dissolved in an appropriate amount of water to obtain a nickel salt solution of 0.10 g nickel / ml. Add an appropriate amount of nickel salt solution into the alumina sol, age at 60° C. for 24 hours, and then dry to obtain the corresponding catalyst precursor. The catalyst precursor was calcined at 400°C for 4 hours to obtain oxidized NiO / Al 2 o 3 catalyst. The catalyst was reduced at 450°C for 12 hours in a pure hydrogen flow of 1.5 L / min to obtain metallic Ni / Al 2 o 3 catalyst.
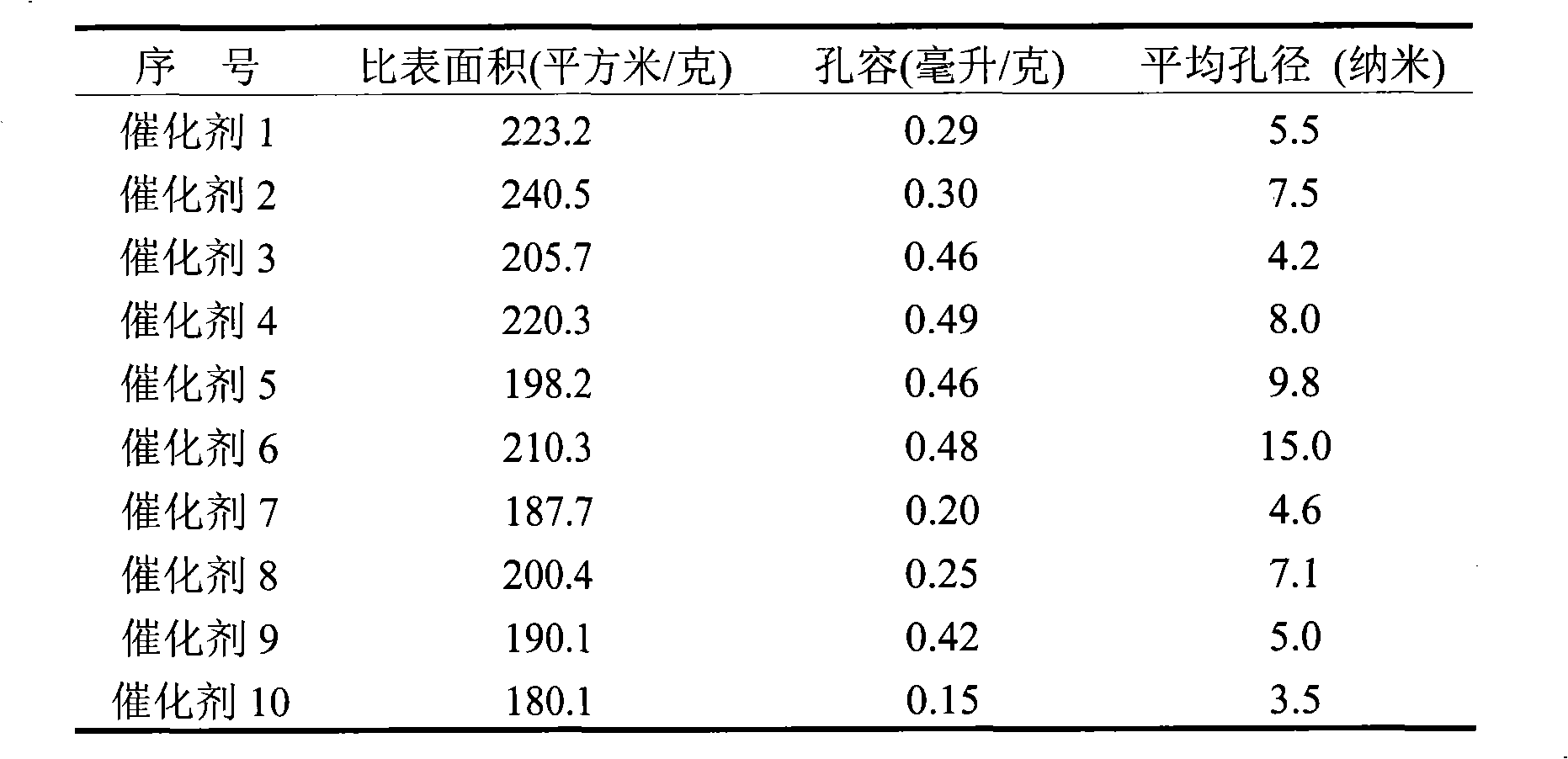
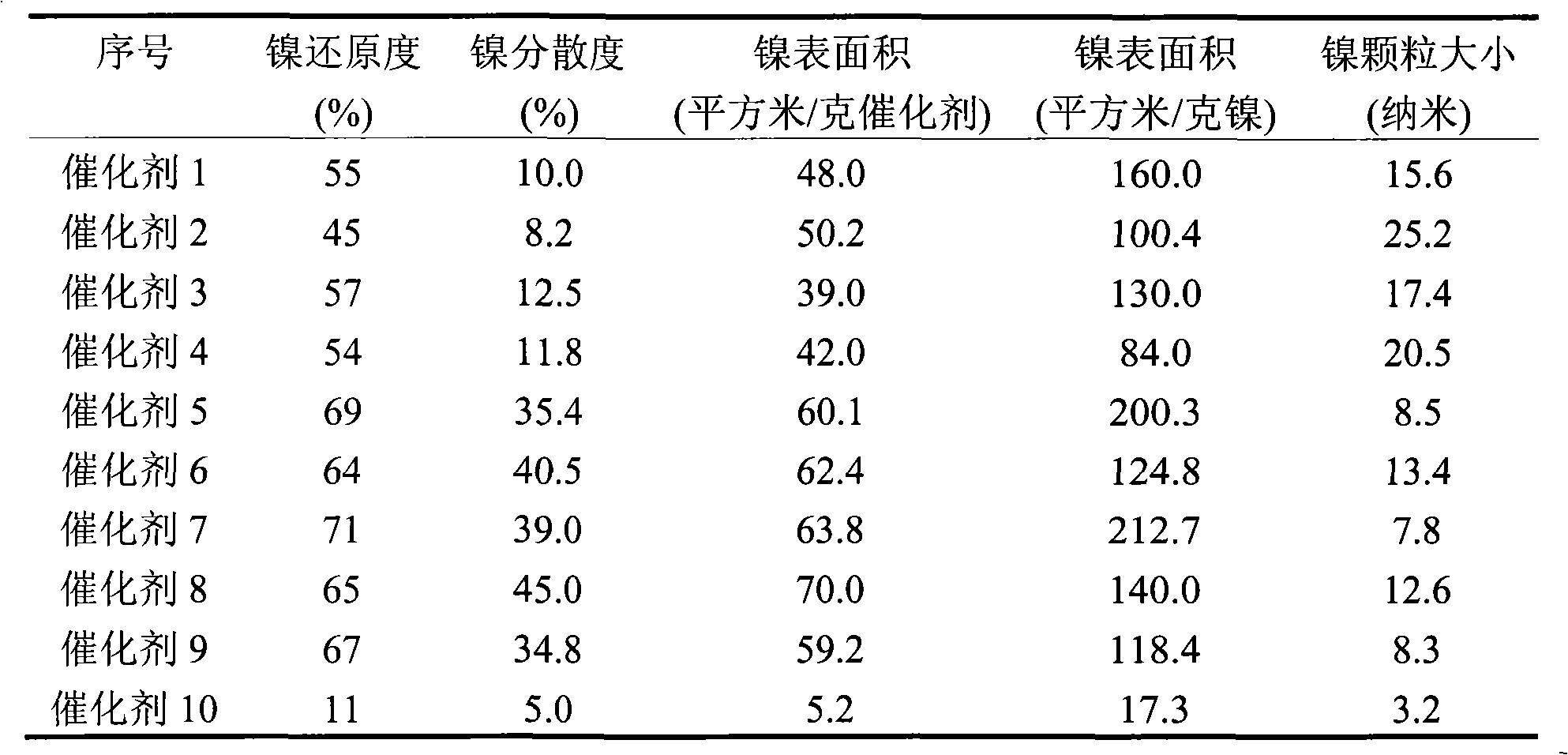
[0013] Change the amount of nickel salt solution added to the alumina sol to obtain catalysts 1-2 in sequence, see Table 1. The physical property data of corresponding catalysts obtained by nitrogen physical adsorption are shown in Table 2. The catalyst reduction degree, dispersion degree and active ...
Embodiment 2
[0021] The preparation and operating conditions of the catalyst were the same as in Example 1, except that the precursor of the nickel salt solution was changed to nickel acetate, and the concentration of the nickel salt solution was 0.04 g nickel / ml. Catalysts 3-4 were sequentially obtained according to the amount of nickel salt solution added to the alumina sol, see Table 1. The physical property data of corresponding catalysts obtained by nitrogen physical adsorption are shown in Table 2. The catalyst reduction degree, dispersion degree and active surface area data are shown in Table 3.
Embodiment 3
[0023] With nitric acid in [H + ] / [AlOOH] molar ratio of 0.25, the pseudo-boehmite powder was peptized for 24 hours to obtain an alumina sol with a solid content of 5% alumina. In terms of molar ratio, basic nickel carbonate:ammonia water:ammonium carbonate=1:6.0:1.5, add an appropriate amount of water to obtain a nickel-ammonia complex solution of 0.10 g nickel / ml. Add the nickel ammonia complex solution to the alumina sol, heat and decompose the complexed nickel ions at 95° C. for 8 hours, and then dry or filter to obtain the corresponding catalyst precursor. The catalyst precursor was calcined at 400°C for 4 hours to obtain oxidized NiO / Al 2 o 3 catalyst. The catalyst was reduced at 450°C for 12 hours in a pure hydrogen flow of 1.5 L / min to obtain metallic Ni / Al 2 o 3 catalyst.
[0024] Change the amount of nickel-ammonia complex solution added to the alumina sol to obtain catalysts 5-6 in sequence, see Table 1. The physical property data of corresponding catalysts o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com