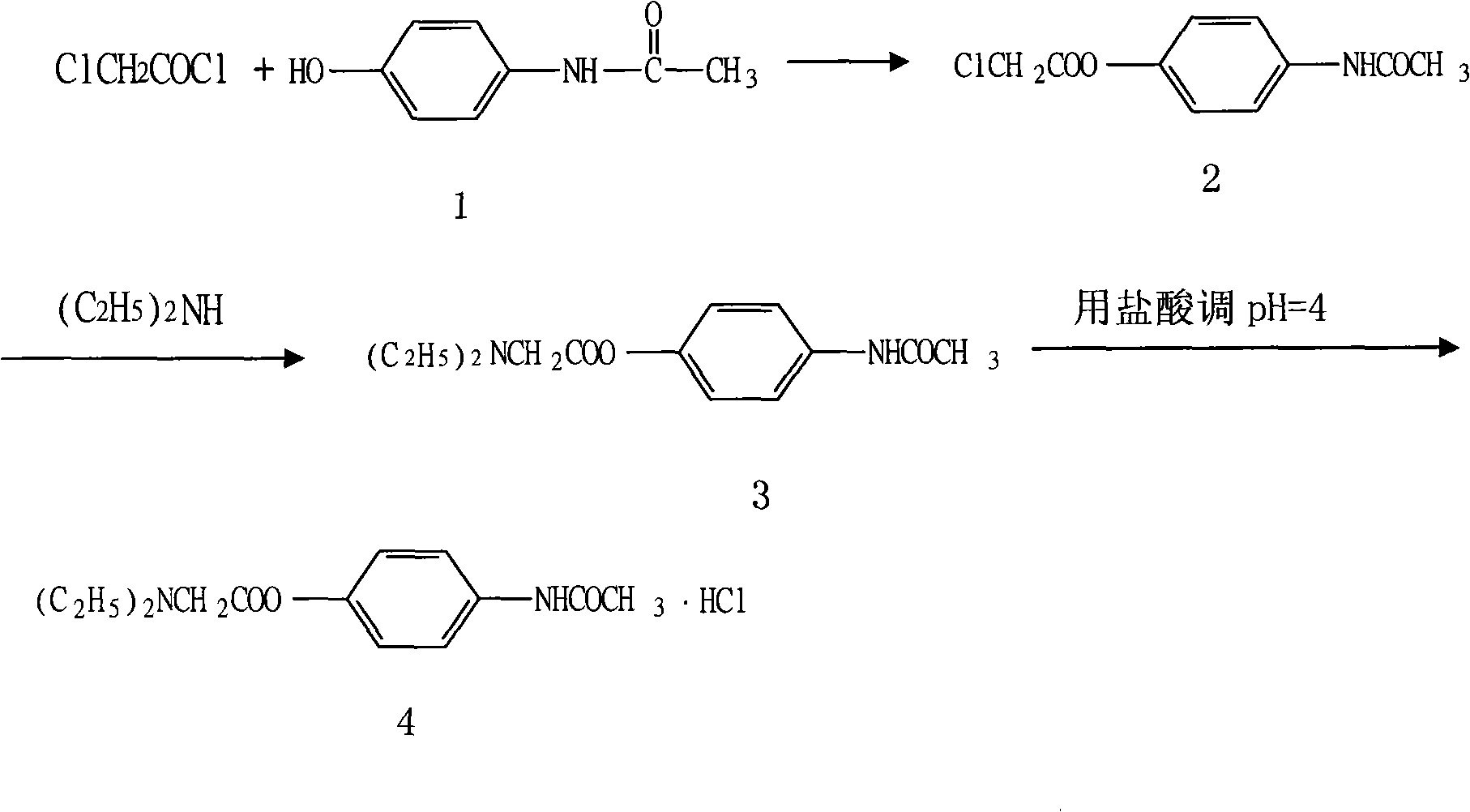
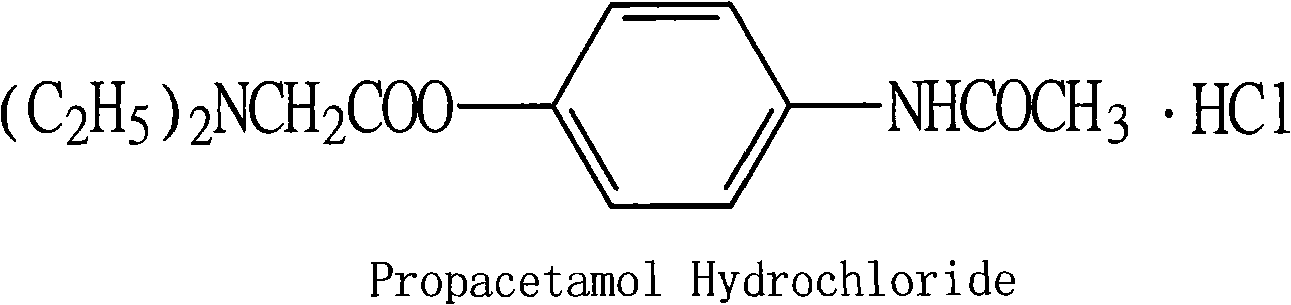
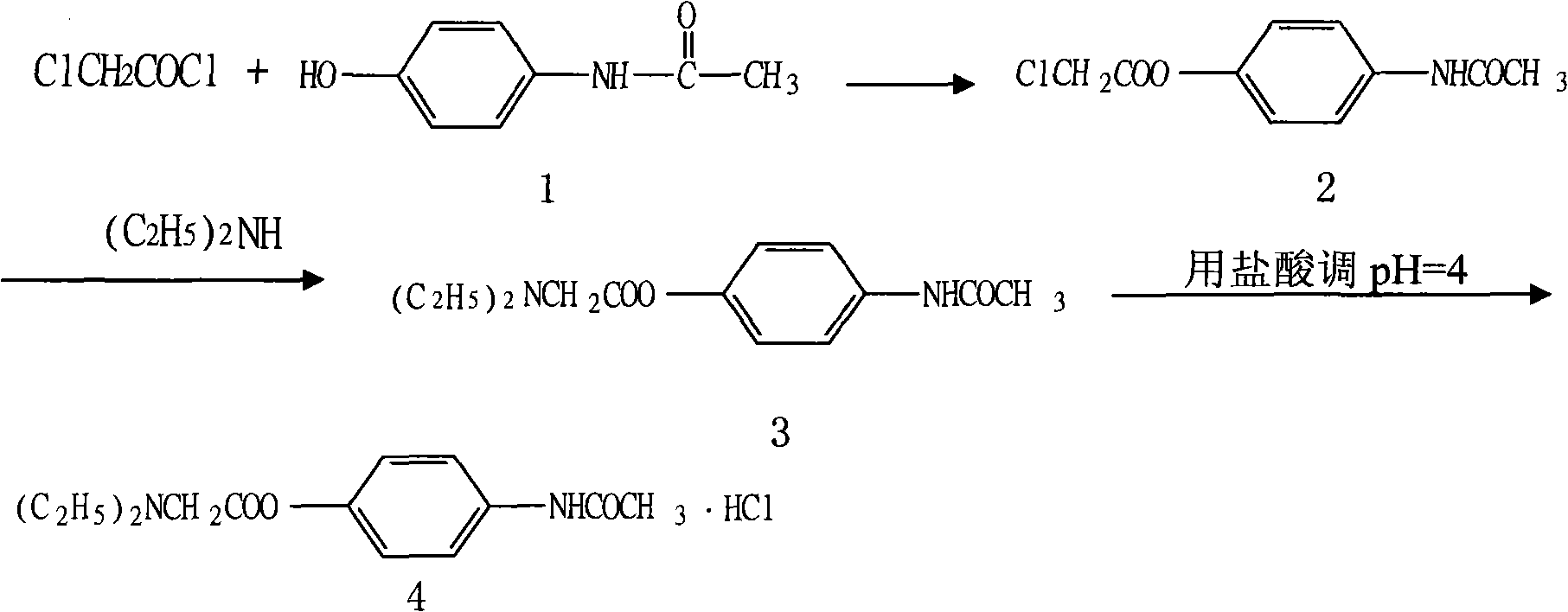
Preparation of propacetamol hydrochloride
A technology of propadamole hydrochloride and compound, which is applied in the field of preparation of pharmaceutical compounds, can solve problems such as difficult production, harsh reaction conditions, and difficult purification, and achieve an increase in total product yield, mild reaction conditions and easy control, and simple purification operations Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1: the preparation of chloroacetic acid-4-acetamidophenyl ester
[0019] In a dry and clean 5000ml three-neck round bottom flask, add 604g of paracetamol (4mol), anhydrous potassium carbonate 198g (2mol), tetrahydrofuran 2000ml (24.6mol), ice bath cooling, slowly add chlorine dropwise under stirring Acetyl chloride 304ml (4mol), keep the temperature of the reaction mixture below 10°C. After the dropwise addition was completed, stir at room temperature for 2 hours, and TLC monitored the reaction (developing agent: absolute ethanol: ethyl acetate=10: 10). After the reaction was completed, it was poured into 3000ml of ice water, and a large amount of near-white precipitate was separated out, and was filtered by suction to obtain The white solid was recrystallized from ethanol after drying slightly to obtain 720 g (3.16 mol) of white needle-like solid, with a yield of 79.0%. mp: 185-186.
Embodiment 2
[0020] Embodiment 2: the preparation of N, N'-diethylglycine 4-acetamidophenyl ester
[0021] In a dry and clean 10000ml three-necked round-bottomed flask, add diethylamine (1248ml, 24.96mol) and stir under an ice-water bath, slowly add 720g (3.16mol) chloroacetic acid-4-acetamidophenyl to it in portions And vigorously stirred, the solution was light yellow, after the addition was completed, the reaction was stirred at 15°C for 2 hours, and the reaction was identified by TLC (developing solvent: absolute ethanol: ethyl acetate = 10:5). After the reaction, the excess diethylamine was recovered by concentration under reduced pressure to obtain 740 g (2.8 mol) of light yellow transparent oil, with a yield of 88.6%. Embodiment 3: the preparation of Propatamole Hydrochloride
Embodiment 3
[0022] Add 740g (2.8mol) of N,N'-diethylglycine 4-acetamidophenyl ester, 30ml of ice water and 2000ml of acetone into a 5000ml round bottom flask. 3.5-4.5, filtered with suction to obtain a near white solid, which was recrystallized with an appropriate amount of absolute ethanol after a little drying to obtain 784g (2.6mol) of white needle crystals, yield 92.85%, mp: 227-228°C. One point was identified by TLC (developing solvent: absolute ethanol: sodium carbonate).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com