7-amino-3-non-3-cephalosporin-4-carbosylic acid preparation method
A cephalosporin and amino technology, which is applied in the field of preparation of 7-amino-3-non-3-cephalosporin-4-carboxylic acid, can solve the problems of high metal ion residues, poor product appearance, harsh use conditions, etc., and achieve reduction The production cost, the process is simple and easy, and the effect of improving product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
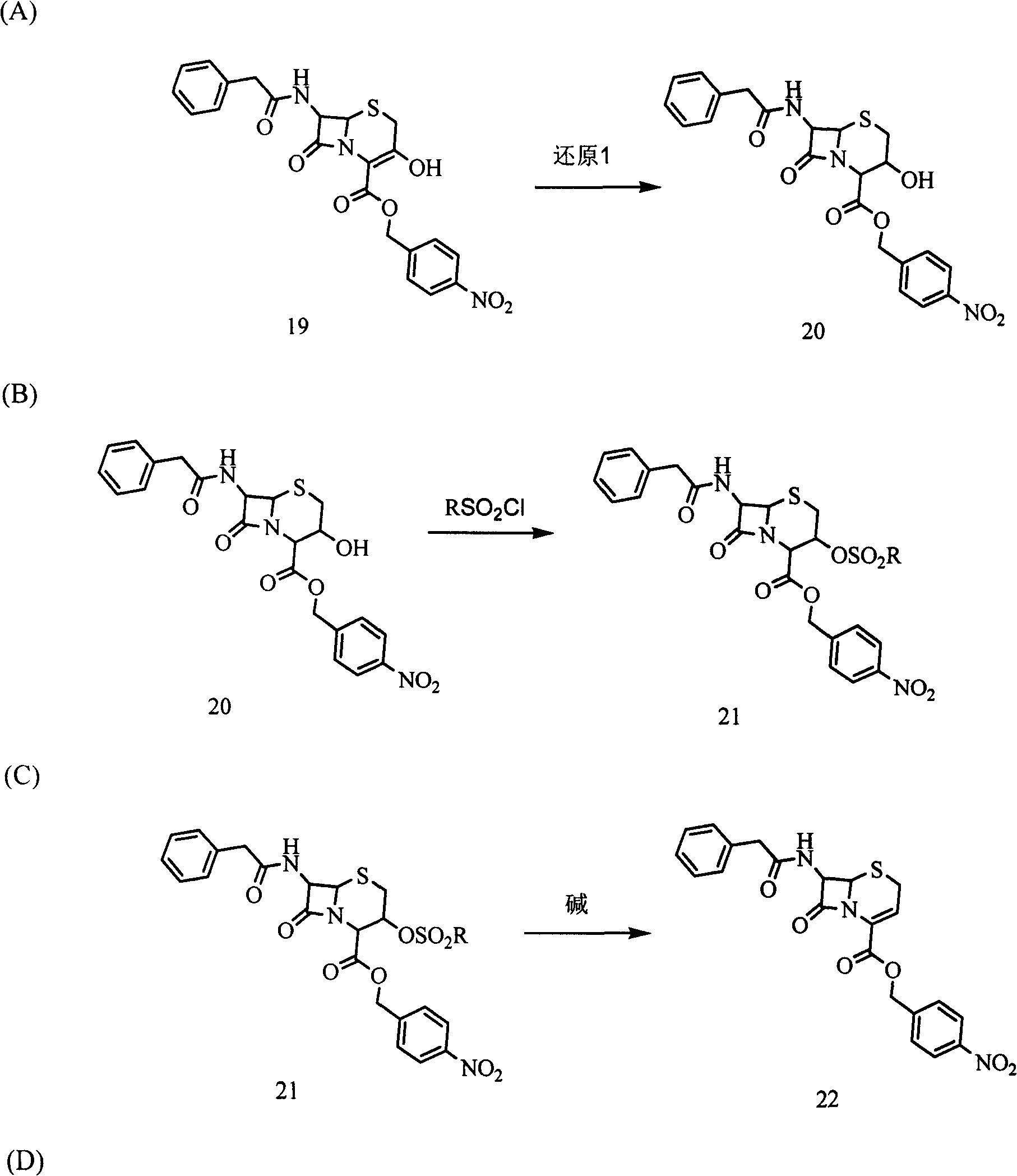
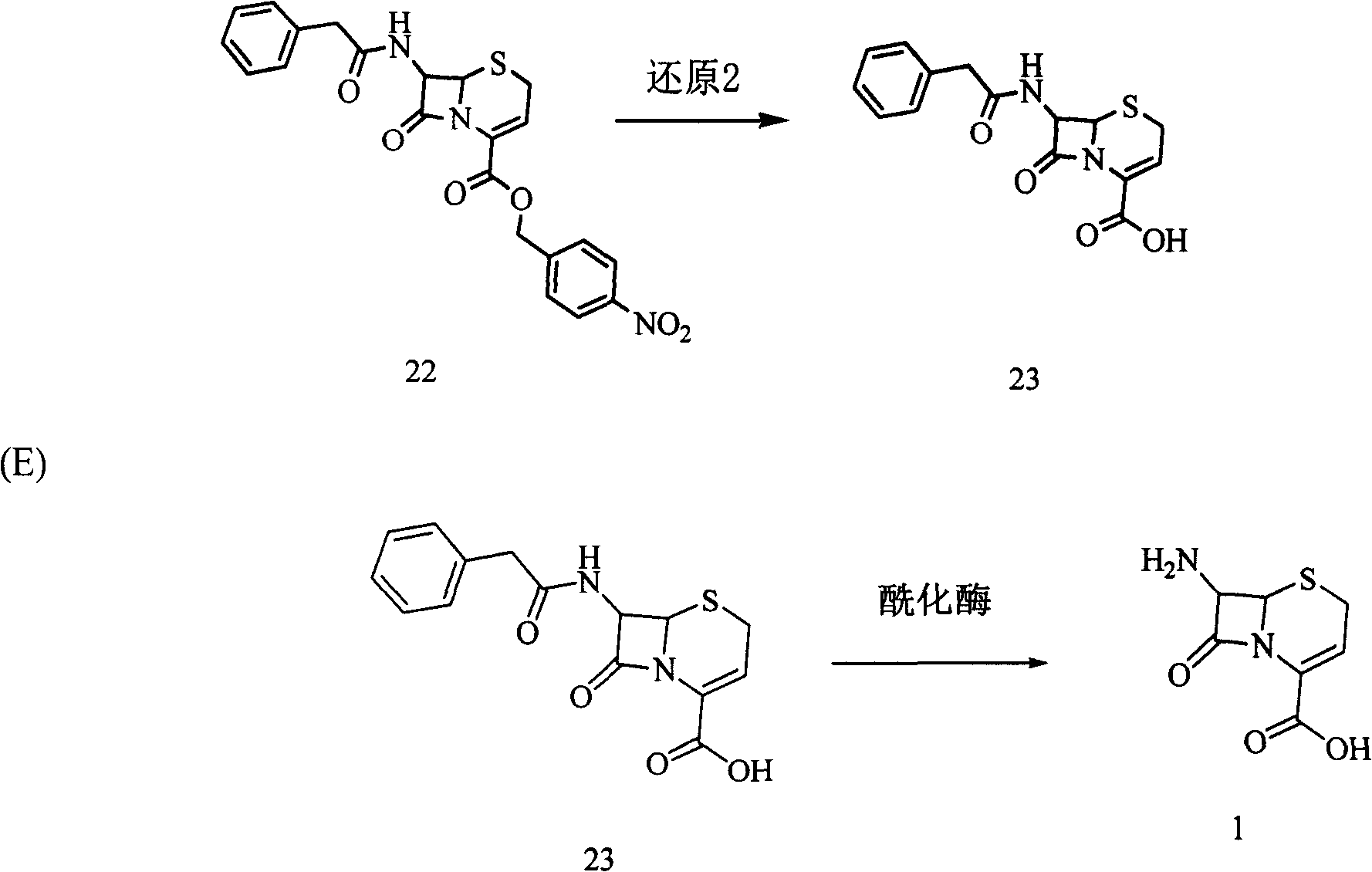
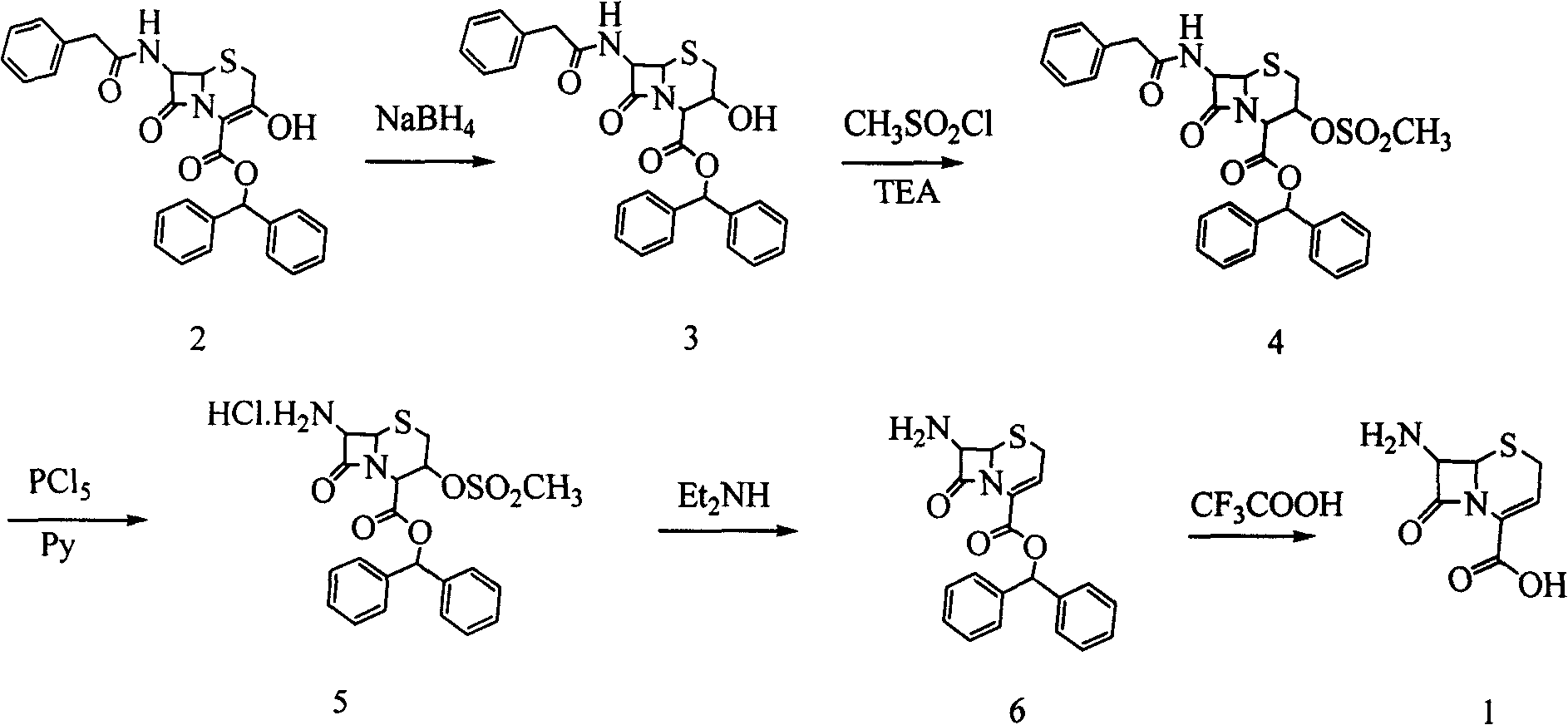
[0037] Weigh 46.9 g of 3-hydroxycephalosporin compound 19 (0.1 mol) in a 500 ml three-neck flask (with thermometer and mechanical stirring), and add 200 ml of tetrahydrofuran to dissolve it. The temperature was lowered to -30°C, and 5.4 g of potassium borohydride (0.1 mol) was added. Continue to cool down to -60°C, and slowly add 100ml of ethanol dropwise. After the dropwise addition, the reaction was continued for 1 hour, a sample was taken for HPLC detection, and the content of the reaction raw material 19 was controlled to be ≤0.5%. 400ml of purified water was added, the temperature was slowly raised to room temperature, and a solid was precipitated. The tetrahydrofuran solvent was recovered under reduced pressure at room temperature. After filtration and drying, 42.39 g of white solid product 20 was obtained (90% molar yield, 98% content by HPLC area normalization method).
[0038]Add 42.3 g of the above-mentioned product 20 (0.09 mol) into a 1000 ml three-necked flask,...
Embodiment 2
[0043] Weigh 46.9 g of 3-hydroxycephalosporin compound 19 (0.1 mol) in a 500 ml three-neck flask (with thermometer and mechanical stirring), add 200 ml of dichloromethane to dissolve. The temperature was lowered to -30°C, and 4.18 g of sodium borohydride (0.11 mol) was added. Continue to cool down to -55°C, and slowly add 100ml of methanol dropwise. After the dropwise addition, the reaction was continued for 1 hour, a sample was taken for HPLC detection, and the content of the reaction raw material 19 was controlled to be ≤0.5%. 400ml of purified water was added, the temperature was slowly raised to room temperature, and a solid was precipitated. The dichloromethane and methanol solvents were recovered under reduced pressure at room temperature. After filtration and drying, 41.5 g of white solid product 20 was obtained (88% molar yield, 98.5% content by HPLC area normalization method).
[0044] Add 41.4 g of the above-mentioned product 20 (0.088 mol) into a 1000 ml three-ne...
Embodiment 3
[0046] Weigh 46.9 g of 3-hydroxycephalosporin compound 19 (0.1 mol) in a 500 ml three-neck flask (with thermometer and mechanical stirring), add 100 ml of dichloromethane and 100 ml of tetrahydrofuran to dissolve. The temperature was lowered to -30°C, and 5.4 g of potassium borohydride (0.1 mol) (0.11 mol) was added. Continue to cool down to -60°C, and slowly add 100ml of ethanol dropwise. After the dropwise addition, the reaction was continued for 1 hour, a sample was taken for HPLC detection, and the content of the reaction raw material 19 was controlled to be ≤0.5%. 400ml of purified water was added, the temperature was slowly raised to room temperature, and a solid was precipitated. The dichloromethane and tetrahydrofuran solvents were recovered under reduced pressure at room temperature. After filtration and drying, 43 g of white solid 20 was obtained (91.3% molar yield, 98.7% content by HPLC area normalization method).
[0047] Add 43 g of the above-mentioned product ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com