Norabieta cantharidin derivant and synthesis thereof
A technology for demethylcantharidin and a synthesis method is applied in the fields of demethylcantharidin derivatives and their synthesis, heterocyclic compounds and their synthesis, and can solve the problems of difficult liver targeting, low encapsulation rate and the like , to achieve the effect of reducing dosage, low production cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
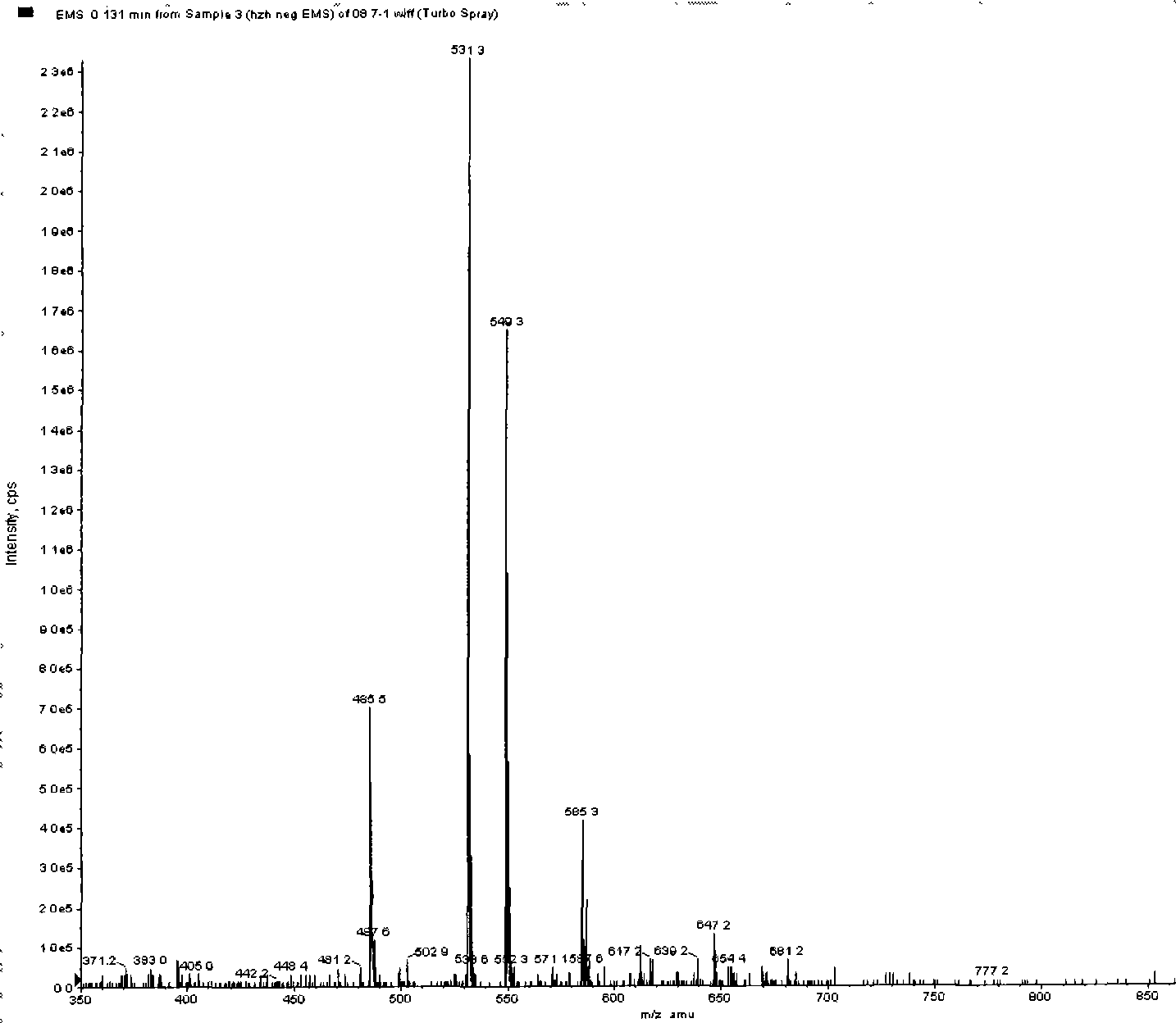
Examples
Embodiment 1
[0024] step 1:
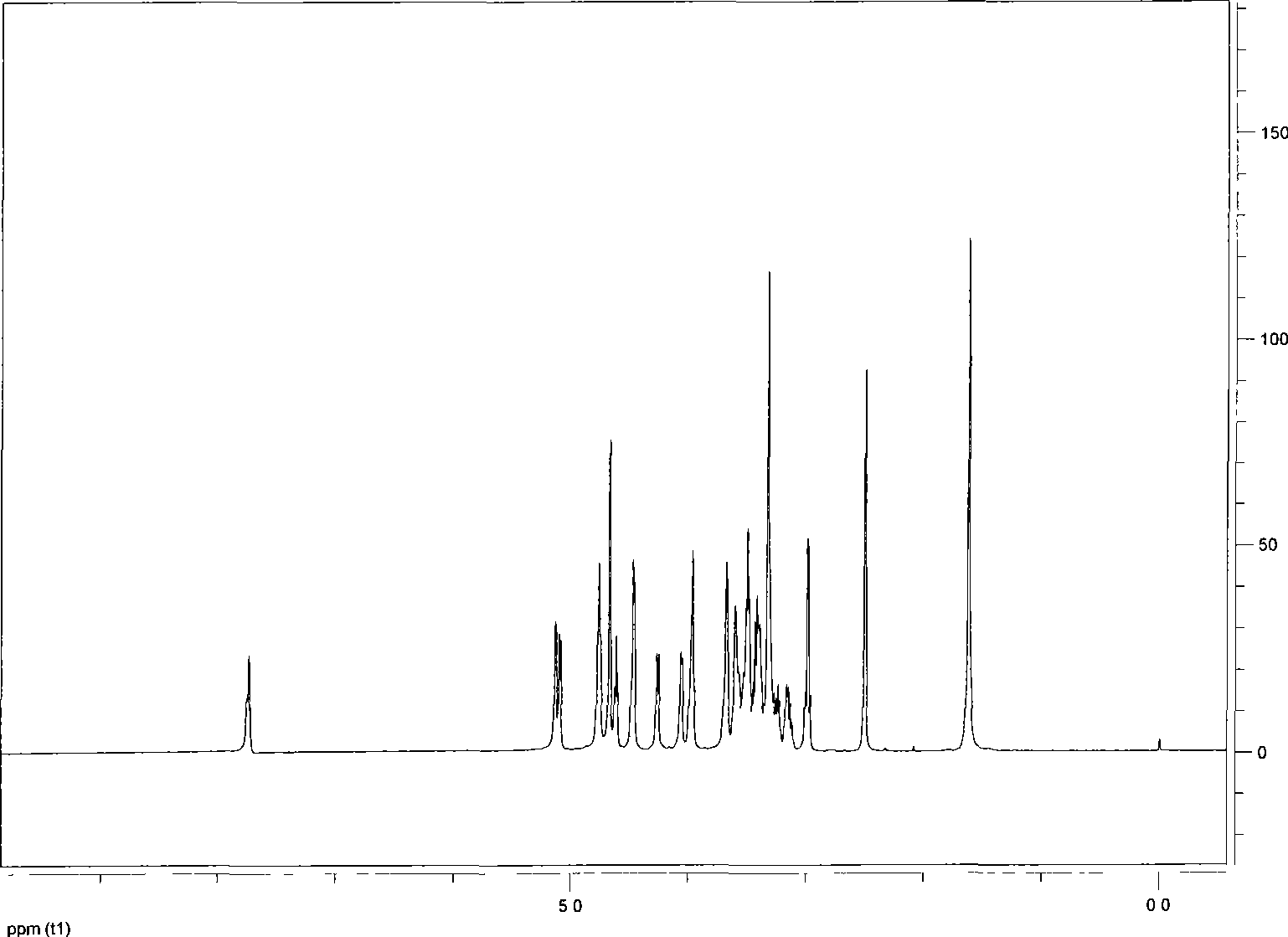
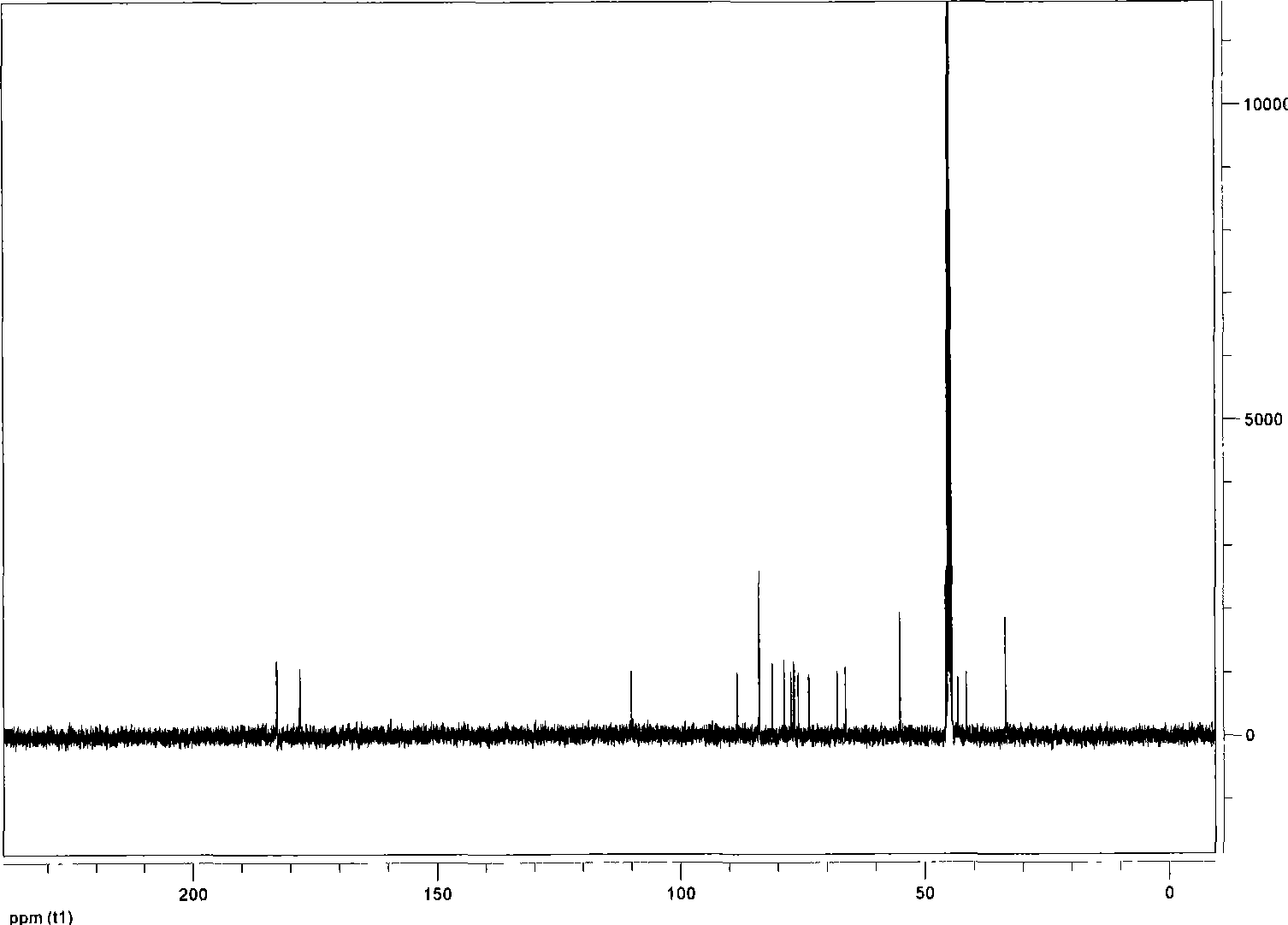
[0025] Anhydrous ethylenediamine (0.8ml, 12mmol) was added dropwise to a methanol solution of norcantharidin (2g, 12mmol), dissolved in an ice bath for 5 minutes, stirred at room temperature for 6 hours, left to stand, filtered, and the filtrate was separated through a column to obtain 1.86 g (8.9 mmol; yield: 73.89%) of white powder, which is norcantharidin monoamide. Melting point: 154-155°C TLC: Rf≈0.5 (ethyl acetate:methanol=4:1); infrared (v / cm -1 ): 3404 (doublet, -NH 2 ), 1689 (singlet-C=O).
[0026] The reaction formula of this step is as follows:
[0027]
[0028] Step 2:
[0029] The sodium lactobionate aqueous solution was passed through a cation exchange column to obtain an aqueous lactobionic acid solution, which was then vacuum-dried at 80°C to obtain white lactobionic acid powder. Its reaction formula is as follows:
[0030]
[0031] Step 3:
[0032] The lactobionic acid (5g, 13.95mmol) obtained in the above steps is dissolved in 26...
Embodiment 2
[0046] step 1:
[0047] Anhydrous ethylenediamine (8ml, 120mmol) was added dropwise into methanol of norcantharidin (20g, 120mmol), dissolved in an ice bath for 10 minutes, stirred at room temperature for 8 hours, allowed to stand, filtered, and the filtrate was separated by column to obtain a white powder 18.9g (90mmol; yield: 74.68%), namely norcantharidin monoamide. Melting point: 154-155°C; TLC: Rf≈0.5 (ethyl acetate:methanol=4:1); infrared (v / cm -1 ): 3403 (doublet, -NH 2 ), 1688 (singlet-C=O).
[0048] Step 2:
[0049] The sodium lactobionate aqueous solution was passed through a cation exchange column to obtain an aqueous lactobionic acid solution, which was then vacuum-dried at 80°C to obtain white lactobionic acid powder.
[0050] Step 3:
[0051] Lactobionic acid (50g, 139.5mmol) was dissolved in 260ml of ethylene glycol methyl ether at a temperature of 110°C, heated and stirred in an oil bath to dissolve, diluted with 130ml of toluene, concentrated by distillat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com