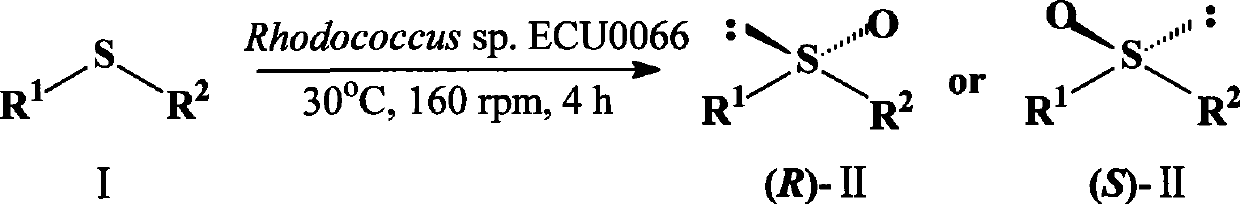
A strain of Rhodococcus and use thereof for preparing optical pure chiral sulphoxide
A technology of rhodococcus and pure benzyl sulfoxide, which is applied in the field of rhodococcus and its use in the production of optically pure chiral sulfoxide, can solve the problems of low ee value of the product and difficulty in meeting the purity requirements, and achieve good catalysis effect, ease of preparation, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 Screening of bacterial strains
[0034] Prepare enriched medium with the following components: (NH 4 ) 2 SO 4 1.0g / L, KH 2 PO 4 3.0g / L, K 2 HPO 4 6.0g / L, MgSO 40.5g / L, CaCl 2 0.05g / L; another rich medium is prepared, the composition is as follows: glucose 15g / L, yeast extract 5g / L, peptone 5g / L, KH 2 PO 4 1.0g / L, K 2 HPO 4 1.0g / L, MgSO 4 0.5g / L, pH7.0. Take a small amount of soil samples and suspend them in 2ml enrichment medium, add sulfide anisole Tween-80 emulsion or methanol solution as the only carbon source, carry out two rounds of enrichment culture under the condition of 25~40℃, the total It takes 2 to 4 days; perform thin-plate chromatography after the enrichment culture, and semi-quantitatively detect the presence or content of the product benzyl sulfoxide. For the enriched culture solution with obvious thioether oxidation products, take a small amount of dilution and spread it on the rich medium plate to cultivate for 1 to 2 days, and ...
Embodiment 2
[0035] Example 2 Culture of microorganisms
[0036] Medium formula: glucose 15g / L, yeast extract 5g / L, peptone 5g / L, KH 2 PO 4 1.0g / L, K 2 HPO 4 1.0g / L, MgSO 4 0.5g / L, pH7.0, high temperature sterilization at 121℃ for 20min.
[0037] Take the Rhodococcus slant preserved at 4°C, pick a ring and inoculate it into a 250ml shake flask containing 50ml of culture medium. Cultivate at 160rpm for 12h at 30°C, transfer to a 500ml shake flask containing 100ml medium at a 5% (v / v) inoculum size, continue to cultivate at 30°C and 160rpm for 30h, and harvest the cells by centrifugation. The measured enzyme activity of the fermentation broth is about 34U / L, the cell concentration is about 30g (wet weight) / L, and the enzyme activity of the unit wet cell is 1.0U / g wet cell.
[0038] The cell enzyme activity unit is defined as the amount of cells required to catalyze the oxidation of thioanisole to produce 1.0 μmol of phenylsulfoxide per minute under the conditions of 30°C and pH 8.0.
Embodiment 3
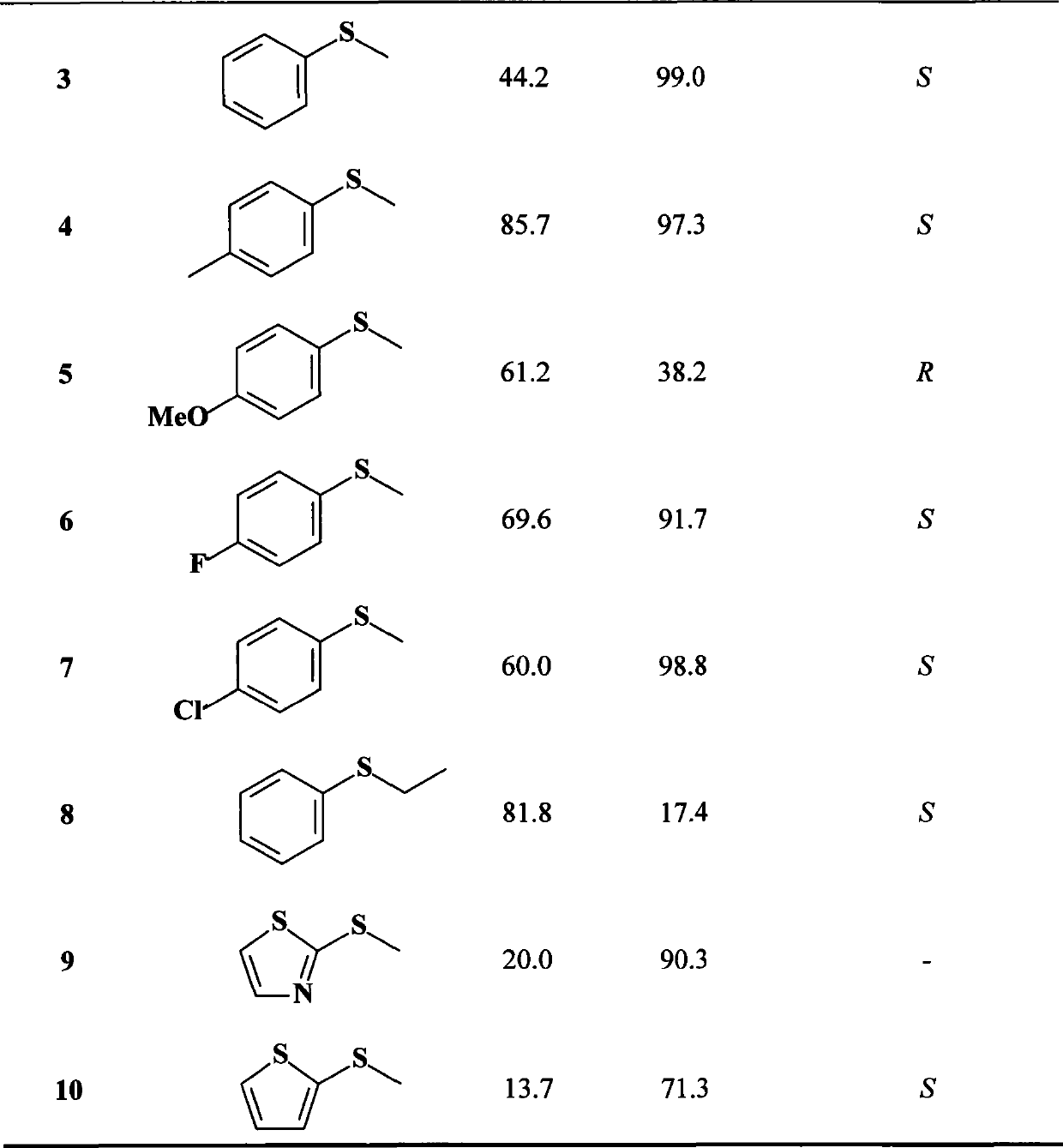
[0039] Example 3 Preparation of (S)-(-)-Benzyl Sulfoxide Using Resting Cells
[0040] Suspend 12 g of resting cells of Rhodococcus sp. ECU0066 obtained by centrifugation in 100 ml of phosphate buffer (50 mM, pH 8.0), add the substrate sulfide anisole, and make the final concentration 5 mM, and set the temperature at 30 ° C and 160 rpm After shaking and reacting on a constant temperature shaker for 4 hours, the reaction solution was centrifuged at 12,000×g for 10 minutes to remove cells. NaCl was added to the supernatant until saturated, extracted with 50 ml of ethyl acetate, and repeated three times. The cells obtained by centrifugation were soaked with 20 ml of ethyl acetate, repeated twice, the two parts of ethyl acetate were combined, and then washed twice with saturated NaCl solution, 10 ml each time. The obtained ethyl acetate extract was dried overnight with anhydrous sodium sulfate, and the ethyl acetate was removed by rotary evaporation to obtain a crude crystalline p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com