Sulfonated aromatic dicarboxilic acid and preparation method thereof
A technology for sulfonating aromatic dicarboxylic acid and disulfonic acid, which is applied in the preparation of sulfonic acid, organic chemistry, etc., can solve the problem of limited types of sulfonated aromatic diacids, few types of commercial aromatic diacid raw materials, and difficulty in meeting application requirements, etc. problem, to achieve the effect of excellent mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
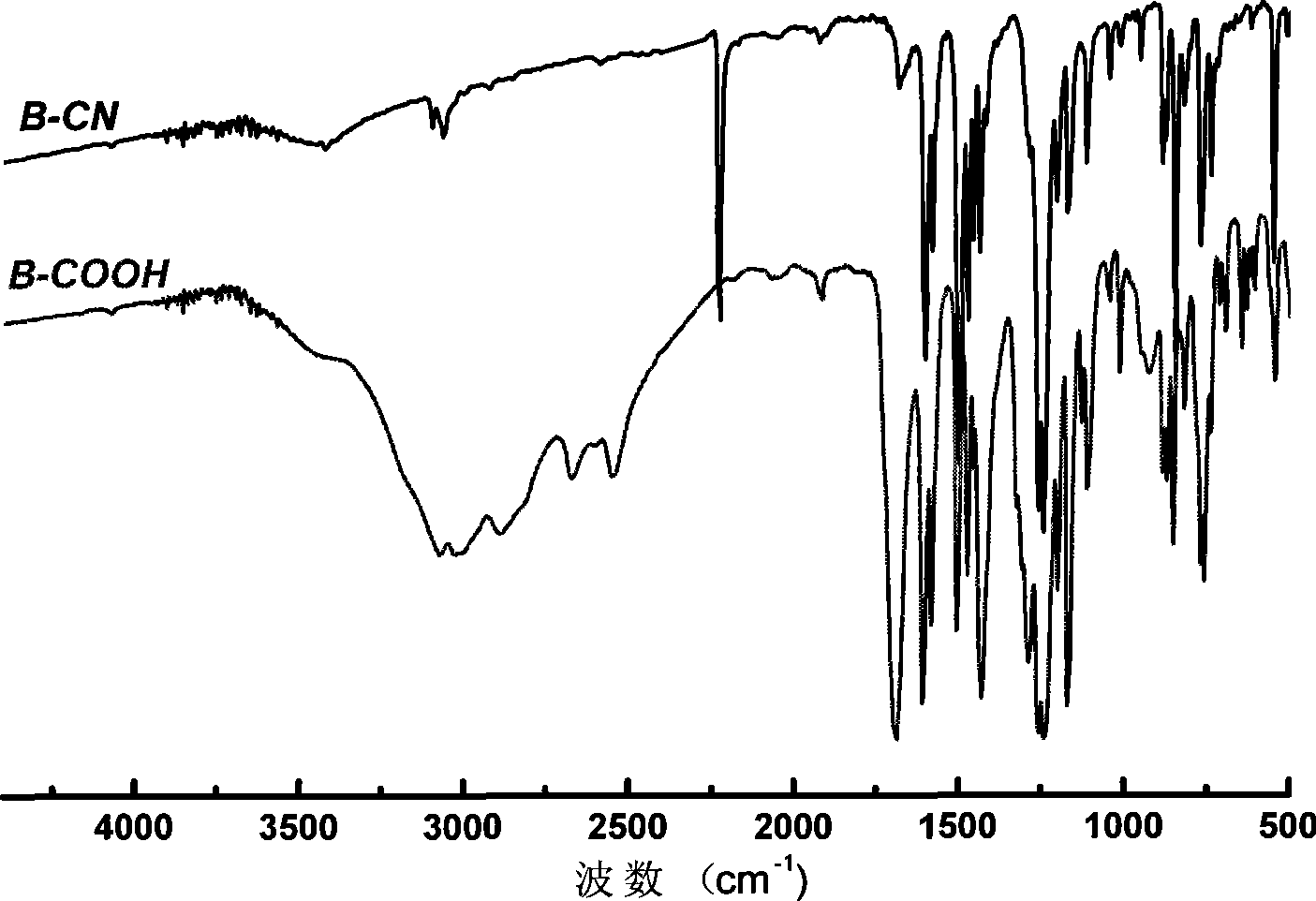
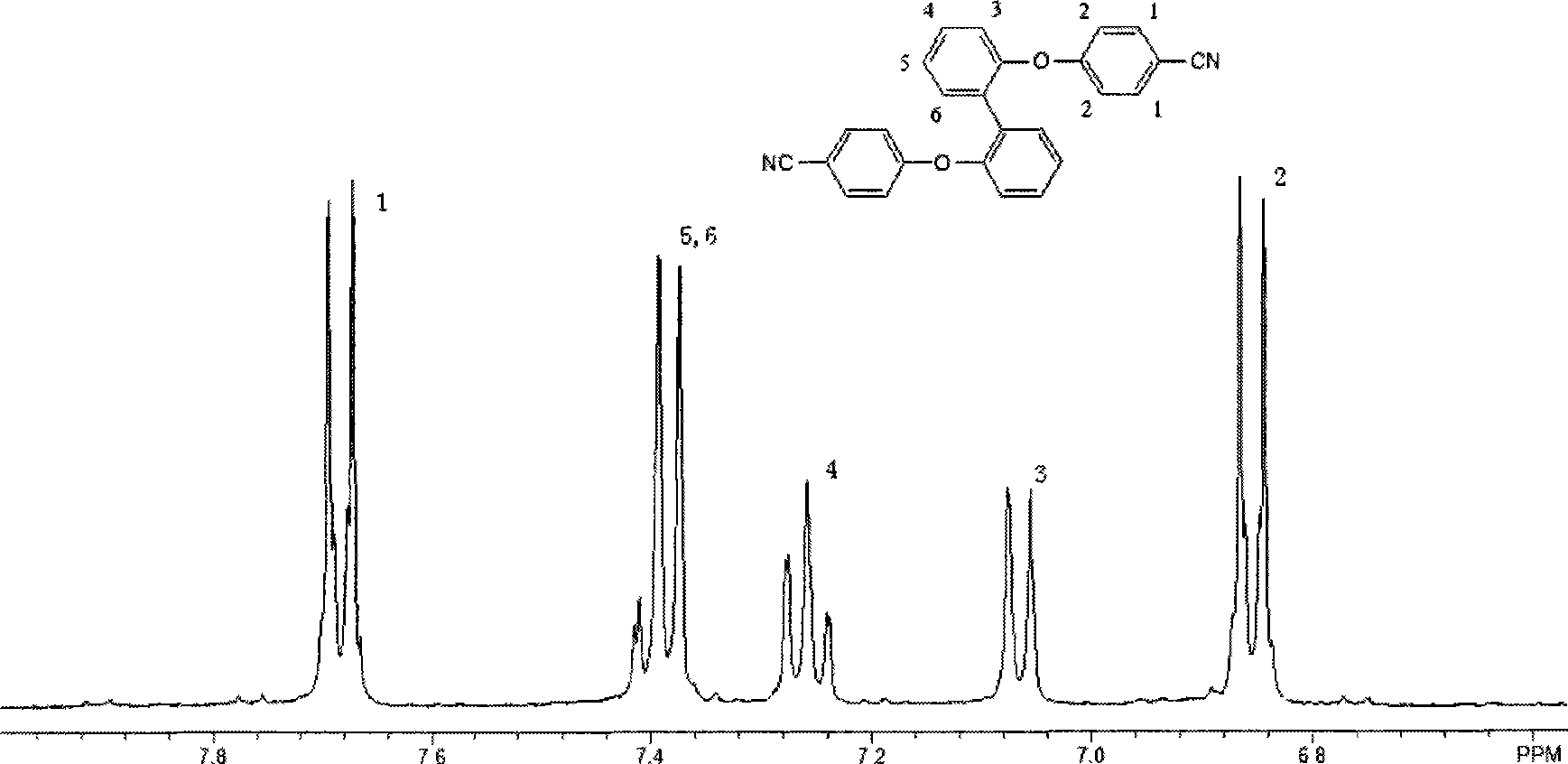
[0024] 5.59g (30mmol) 2,2'-diphenol, 7.27g (60mmol) 4-fluorobenzonitrile and 16.6g (120mmol) potassium carbonate were added to 100ml of mechanical stirring, nitrogen inlet and reflux condenser In the reactor, add 30ml of N-methylpyrrolidone and react at 180°C for 20 hours. After cooling down to room temperature, the product is poured into water, filtered, and the product is vacuum-dried to obtain 2,2'-bis(4-nitrile 10.48 g of phenoxy)biphenyl, and the yield was 90%.
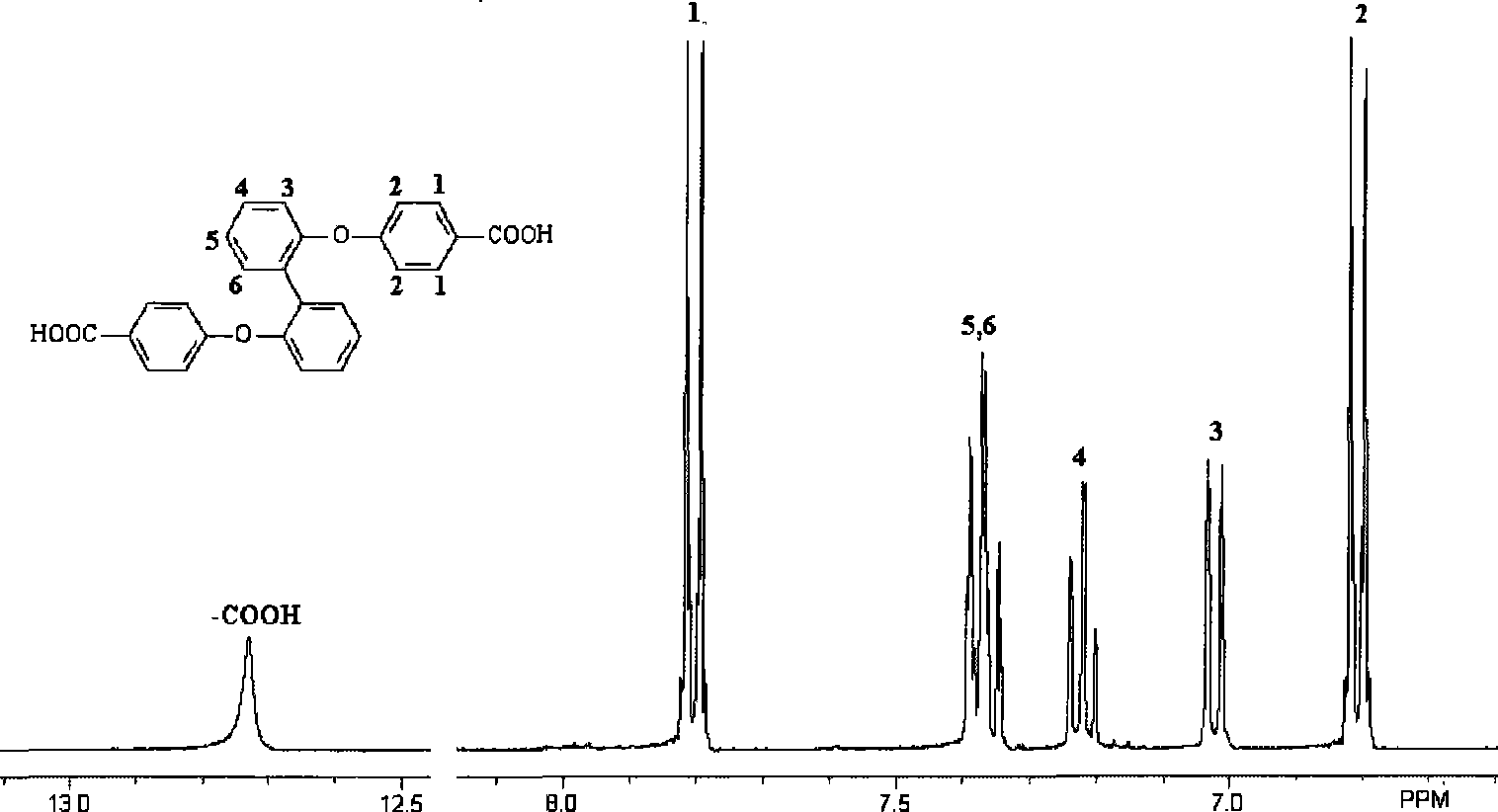
[0025] 10.48g (27mmol) of 2,2'-bis(4-cyanophenoxy)biphenyl, 7.84g (140mmol) of potassium hydroxide and 190ml of ethylene glycol were added to the reactor and reacted at 180°C for 20 hour, after cooling down to room temperature, the reaction solution was poured into water, added hydrochloric acid to acidic (PH=1), filtered, and the product was vacuum-dried to obtain 2,2'-di(4-carboxyphenoxy)biphenyl 9.77g, producing The rate is 85%.
[0026] Add 9.77g (23mmol) of 2,2'-bis(4-carboxyphenoxy)biphenyl and 135ml of c...
Embodiment 2
[0028] 7.45g (40mmol) 2,2'-diphenol, 9.69g (80mmol) 4-fluorobenzonitrile and 16.6g (120mmol) potassium carbonate were added to 100ml of mechanical stirring, nitrogen inlet and reflux condenser In the reactor, add 60ml of N-methylpyrrolidone and react at 180°C for 24 hours. After cooling down to room temperature, the product is poured into water, filtered, and the product is vacuum-dried to obtain 2,2'-bis(4-nitrile 13.6 g of phenoxy)biphenyl, and the yield was 88%.
[0029] 13.6g (35mmol) of 2,2'-bis(4-cyanophenoxy)biphenyl, 9.8g (175mmol) of potassium hydroxide and 200ml of ethylene glycol were added to the reactor and reacted at 180°C for 20 Hours, after cooling down to room temperature, the reaction solution was poured into water, added hydrochloric acid to acidic (PH=1), filtered, and the product was vacuum-dried to obtain 12.7 g of 2,2'-bis(4-carboxyphenoxy)biphenyl, producing The rate is 85%.
[0030] Add 12.7g (29.8mmol) of 2,2'-bis(4-carboxyphenoxy)biphenyl and 100ml...
Embodiment 3
[0032] 5.59g (30mmol) 4,4'-diphenol, 7.27g (60mmol) 4-fluorobenzonitrile and 16.6g (120mmol) potassium carbonate were added to 100ml of mechanical stirring, nitrogen inlet and reflux condenser In the reactor, add 30ml of N-methylpyrrolidone, react at 180°C for 20 hours, and after cooling down to room temperature, pour the product into water, filter, and dry the product in vacuum to obtain 4,4'-bis(4-nitrile 9.9 g of phenoxy)biphenyl, and the yield was 85%.
[0033]Add 9.9g (25.5mmol) of 4,4'-bis(4-cyanophenoxy)biphenyl, 7.28g (130mmol) of potassium hydroxide and 180ml of ethylene glycol into the reactor and react at 180°C After 20 hours, after cooling down to room temperature, the reaction solution was poured into water, hydrochloric acid was added to make it acidic (PH=1), filtered, and the product was dried in vacuum to obtain 8.9 g of 4,4'-bis(4-carboxyphenoxy)biphenyl, The yield was 82%.
[0034] Add 8.9g (21mmol) of 4,4'-bis(4-carboxyphenoxy)biphenyl and 120ml of concen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com