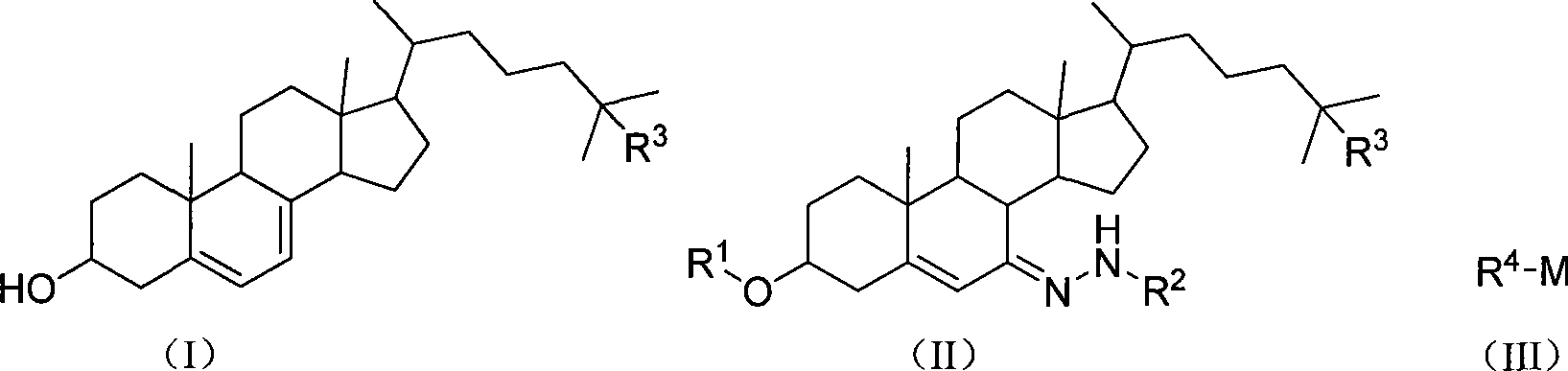
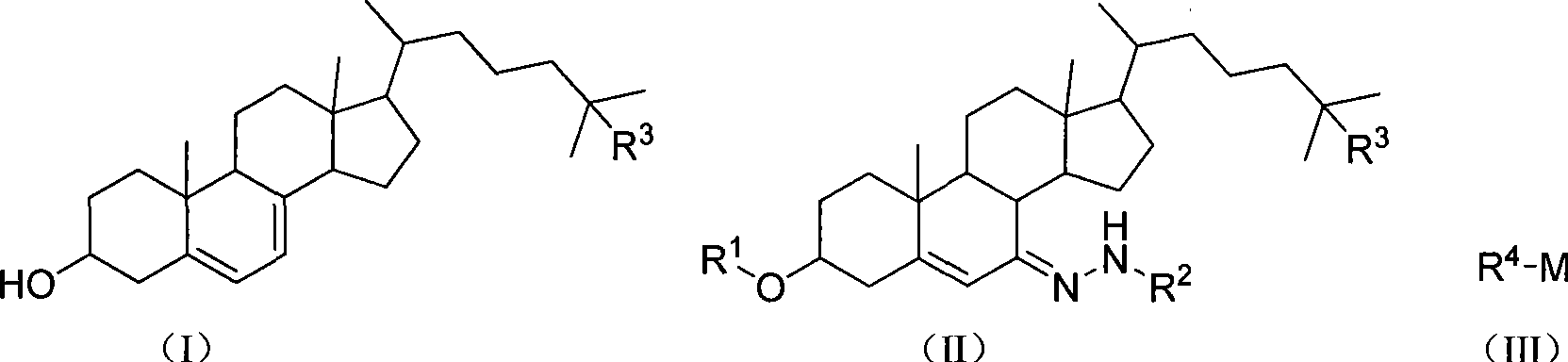
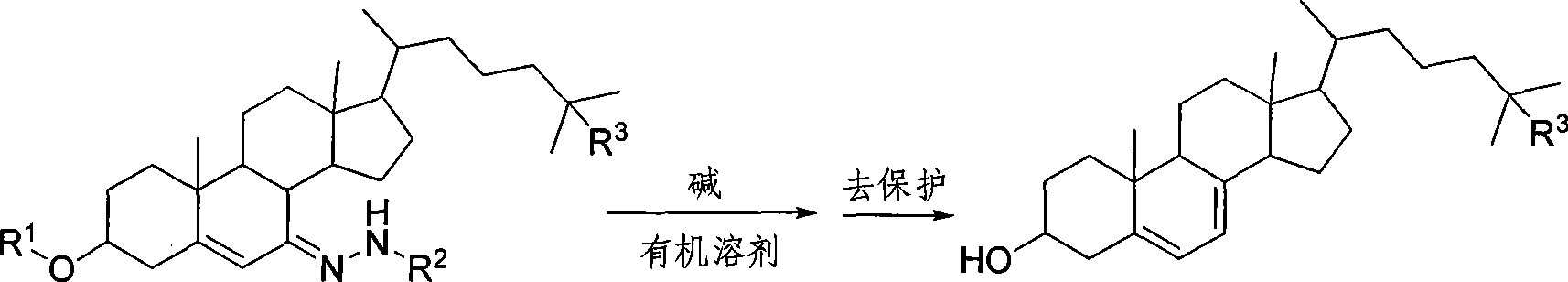
Chemical synthesis method of 5,7-diene steroids compounds
A technology of diene steroids and a synthesis method, which is applied in 5 fields, can solve the problems of affecting the photochemical reaction of vitamin D drugs, serious pollution, high price and the like, and achieves low production cost, high reaction yield and mild reaction conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] In a 1000ml four-neck flask equipped with a thermometer, reflux condenser and mechanical stirring, add 100mmol (61g) of 3-acetoxycholesterol-7-p-toluenesulfonylhydrazone, dissolve it in 400mL of toluene, cool to -20°C, add A solution of methyl lithium (6.6 g, 300 mmol) in toluene (50 mL) was maintained at -20°C, reacted for 0.5 hour, then raised to 25°C and stirred for 2 hours. After the reaction is complete, add 100 mL of water and stir thoroughly, add 4 g of sodium hydroxide, heat up to reflux, react for 30 minutes, adjust the pH to 7 with hydrochloric acid or acetic acid, leave the organic phase to dry with anhydrous sodium sulfate after standing for layers, and evaporate the solvent. Recrystallization with ethanol / toluene gave 36.1 g of the target product 5,7-dienyl cholesterol as a light yellow solid with a melting point of 145-146° C. and a yield of 94%.
Embodiment 2
[0030] In a 1000ml four-neck flask equipped with a thermometer, reflux condenser and mechanical stirring, add 100mmol (61g) of 3-acetoxycholesterol-7-p-toluenesulfonylhydrazone, dissolve it in 400mL of toluene, cool to -20°C, add A solution of methyl lithium (6.6 g, 300 mmol) in toluene (50 mL) was maintained at -20°C, reacted for 0.5 hour, then raised to 25°C and stirred for 2 hours. After the reaction is complete, add 100 mL of methanol and stir thoroughly, add 4 g of sodium hydroxide, heat up to reflux, react for 30 minutes, adjust the pH to 7 with hydrochloric acid or acetic acid, evaporate the solvent, and recrystallize with ethanol / toluene to obtain the target product 5,7- 36.6 g of Diene Cholesterol is a light yellow solid with a melting point of 145-146° C. and a yield of 95%.
Embodiment 3
[0032] In a 1000ml four-neck flask equipped with a thermometer, reflux condenser and mechanical stirring, add 100mmol (61g) of 3-acetoxycholesterol-7-p-toluenesulfonylhydrazone, dissolve it in 400mL of toluene, cool to -20°C, add A solution of methyl lithium (6.6 g, 300 mmol) in toluene (50 mL) was maintained at -20°C, reacted for 0.5 hour, then raised to 25°C and stirred for 2 hours. After the reaction is complete, add 100 mL of ethanol and stir thoroughly, add 4 g of sodium hydroxide, heat up to reflux, react for 30 minutes, adjust the pH to 7 with hydrochloric acid or acetic acid, evaporate the solvent, and recrystallize with ethanol / toluene to obtain the target product 5,7- 36.6 g of Diene Cholesterol is a light yellow solid with a melting point of 145-146° C. and a yield of 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com