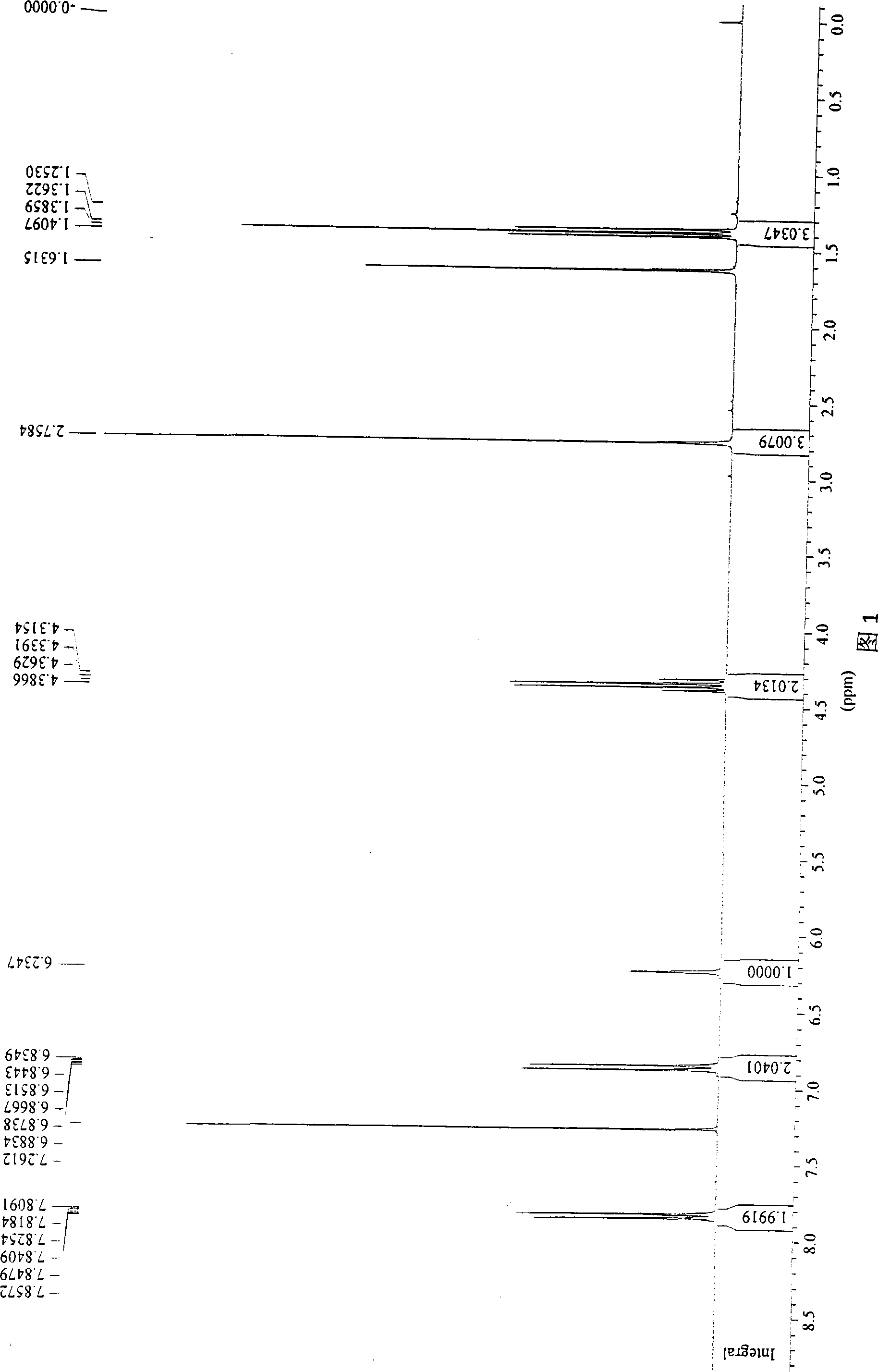
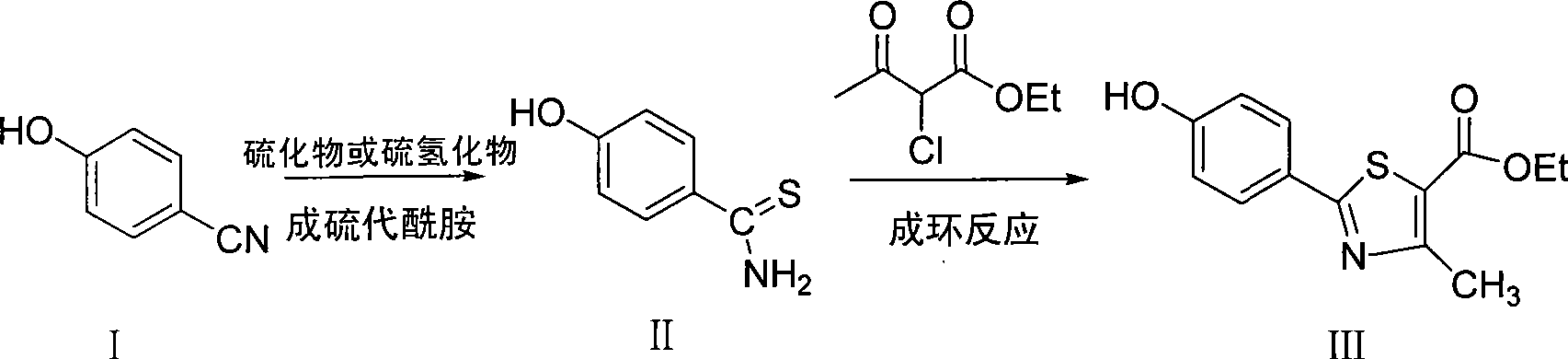
Method for preparing 2-(4-hydroxyl phenyl)-4-methyl-1,3-thiazole-5-carboxylic acid ethyl ester by one pot method
A technology of hydroxyphenyl and ethyl carboxylate, which is applied in the field of preparation of thiazole derivative pharmaceutical intermediates, can solve the problems of difficult operation control, high equipment requirements, complicated purification, etc., and achieve simple operation, high yield and purity, The effect of simple reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Put p-hydroxybenzonitrile (12g, 100mmol), sodium sulfide (7.8g, 100mmol), ferric chloride hexahydrate (13.5g, 50mmol) N, N-dimethylformamide (100mL) on a thermometer, Condensate and reflux and stir in the reactor of the stirring device, stir to dissolve the solid, and stir and react at 25°C for 2 hours. Measure ethyl acetate (150 mL) and ethyl 2-chloroacetoacetate (16.4 g, 100 mmol) into the reaction solution, raise the temperature to 82° C., and react under reflux for 3 hours. After the reaction, 1mol / L hydrochloric acid was added dropwise to adjust the pH of the reaction solution to 6, filtered to remove the precipitate, the filtrate was poured into 5 times the volume of the reaction solution under stirring, a large amount of white floccules precipitated, filtered to obtain a solid before use After washing with a large amount of water twice, after drying, 19.7 g of the target product 2-(4-hydroxyphenyl)-4-methyl-1,3-thiazole-5-ethyl acetate with higher purity can be obtai...
Embodiment 2
[0017] Place p-hydroxybenzonitrile (12g, 100mmol), sodium hydrosulfide (5.6g, 100mmol), ferric chloride hexahydrate (13.5g, 50mmol) pyridine (100mL) in a reaction with a thermometer, reflux and stirring device In the kettle, stir to dissolve the solids, and stir and react at 30°C for 4 hours. Measure chloroform (150 mL) and ethyl 2-chloroacetoacetate (16.4 g, 100 mmol) into the reaction solution, raise the temperature to 65° C., and react under reflux for 5 hours. After the reaction, 3mol / L nitric acid was added dropwise to adjust the pH of the reaction solution to 6.5, and the precipitate was removed by filtration. The filtrate was poured into 5 times the volume of the reaction solution under stirring. A large amount of white flocs precipitated out. The solid was filtered and then used in a large amount. After washing 3 times with water, after drying, 21.0 g of 2-(4-hydroxyphenyl)-4-methyl-1,3-thiazole-5-ethyl acetate, the target product with higher purity, can be obtained, and t...
Embodiment 3
[0020] Put p-hydroxybenzonitrile (12g, 100mmol), sodium sulfide (7.8g, 100mmol), copper chloride dihydrate (12.7g, 75mmol) toluene (100mL) in a reactor equipped with a thermometer, reflux and stirring device , Stir to dissolve the solid, stir and react at 35°C for 2 hours. Measure ethanol (200 mL) and ethyl 2-chloroacetoacetate (16.4 g, 100 mmol) into the reaction solution, raise the temperature to 80° C., and react under reflux for 6 hours. After the reaction, 2mol / L hydrochloric acid was added dropwise to adjust the pH of the reaction solution to 7, and the precipitate was removed by filtration. The filtrate was poured into 5 times the volume of the reaction solution under stirring. A large amount of white floccules precipitated out. The solid was filtered and used again. After washing with a large amount of water for 3 times, after drying, 22.2 g of 2-(4-hydroxyphenyl)-4-methyl-1,3-thiazole-5-ethyl acetate, a target product with higher purity, can be obtained, with a yield of 8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com