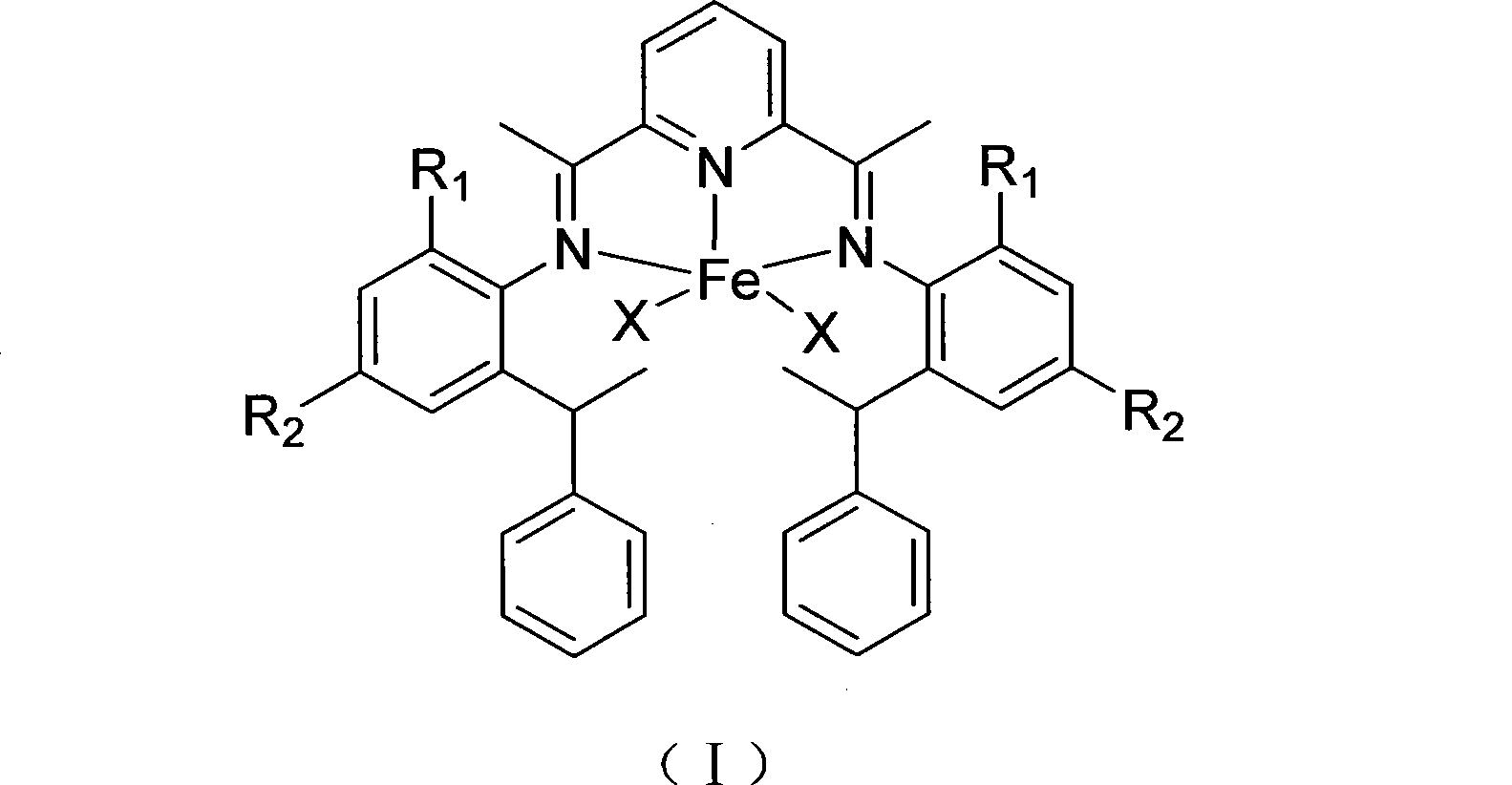
Pyridine diimine iron olefin polymerizing catalyst, as well as preparation method and application thereof
A technique for the polymerization of iron pyridinediimide and olefins, which is applied in the direction of iron organic compounds, etc., which can solve the problems of complex preparation process and short catalytic life of catalysts, and achieve the effects of simple preparation method, increased molecular weight, and extended service life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
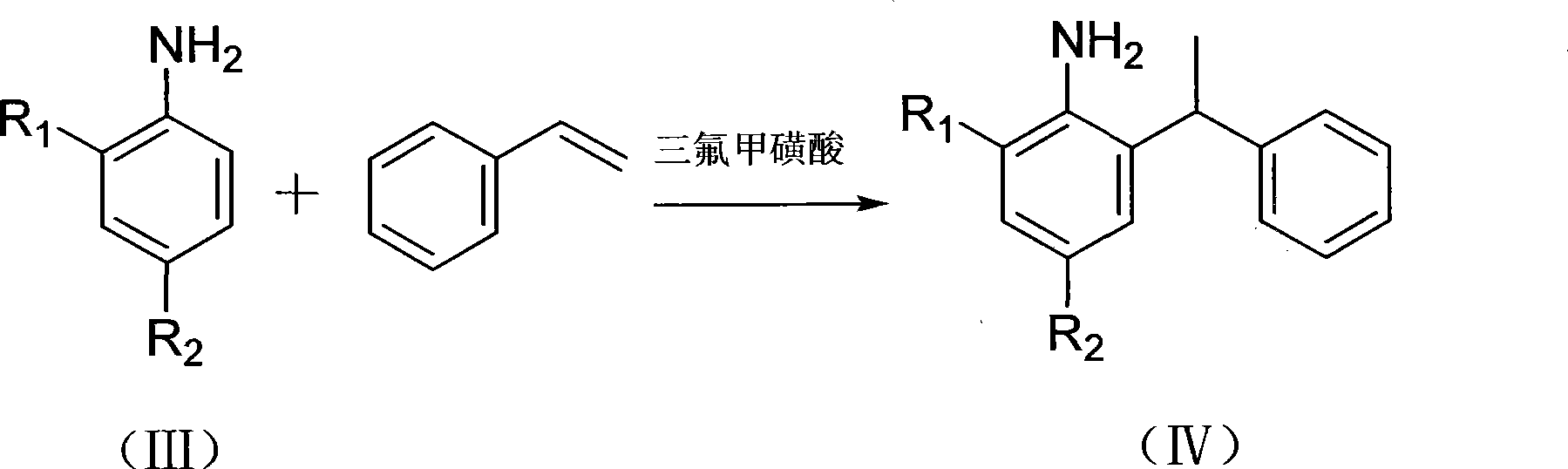
[0043] The asymmetrically substituted amine represented by formula (IV) prepared in this example, wherein R 1 =H,R 2 =CH 3 , the preparation process is as follows:
[0044] Under a nitrogen atmosphere, 15 mL of styrene and 2 mL of trifluoromethanesulfonic acid were injected into a reactor containing 25 ml of p-toluidine xylene solution (containing 13.9 g of p-toluidine xylene), and the reaction was stirred and refluxed at 130 °C. After 30 hours, the product was an orange-red oil, which was separated by a silica gel column, and the eluent was a mixed solvent of petroleum ether:ethyl acetate=20:1 (volume ratio) to obtain 5.31 g of a light red oil, which was The target product of this example has a yield of 19.4%.
[0045] 1 H NMR (300MHz, CDCl 3 ): δ (ppm): 6.9-7.3 (m, 7H, aryl-H), 6.59 (d, 1H, aryl-H), 4.12 (q, 1H, CHCH 3 ), 3.30 (2H, NH 2 ), 2.35(s, 3H, p-CH 3 ), 1.67(d, 3H, CHCH 3 ).
[0046] Mass spectrum EI-MS (m / z): 212.2 [M] + .
Embodiment 2
[0048] The asymmetrically substituted amine represented by formula (IV) prepared in this example, wherein R 1 =CH 3 , R 2 =CH 3 , the preparation process is as follows:
[0049] Under a nitrogen atmosphere, 20 mL of styrene and 3 mL of trifluoromethanesulfonic acid were injected into a reactor containing 25 mL of xylene solvent (containing 15 mL of 2,4-dimethylaniline), and the reaction was stirred and refluxed at 160° C. for 30 hours. The crude product obtained from the reaction was recrystallized with a mixed solvent of petroleum ether and ethyl acetate (v:v=20:1) to obtain 8.43 g of white flocculent crystals, which was the target product of this example, with a yield of 31%.
[0050] 1 H NMR (300MHz, CDCl 3 ): δ (ppm): 7.16-7.30 (m, 5H, aryl-H), 6.99 (s, 1H, aryl-H), 6.84 (s, 1H, aryl-H), 4.12 (q, 1H, CHCH) 3 ), 2.30(s, 3H, p-CH 3 ), 2.13(s, 3H, O-CH 3 ), 1.62 (d, 3H, CHCH3).
[0051]Mass spectrum EI-MS (m / z): 226.2 [M] + .
Embodiment 3
[0053] The asymmetrically substituted amine represented by formula (IV) prepared in this example, wherein R 1 =H,R 2 =OCH 3 , the preparation process is as follows:
[0054] Under a nitrogen atmosphere, 13 mL of styrene and 2 mL of trifluoromethanesulfonic acid were injected into a reactor equipped with 25 ml of xylene solvent (containing 15.7 g of p-methoxyaniline), and the reaction was stirred and refluxed at 160 ° C for 30 hours. It was an orange-red oil, and the product was separated by a silica gel column, and the eluent was a mixed solvent of petroleum ether: ethyl acetate=5:1 (volume ratio) to obtain 3.19 g of white crystals, which was the target product of this example. , the yield is 11%.
[0055] 1 H NMR (300MHz, CDCl 3 ): δ (ppm): 7.18-7.30 (m, 5H, aryl-H), 6.91 (s, 1H, aryl-H), 6.58-6.69 (m, 2H, aryl-H), 4.11 (q, 1H, CHCH 3 ), 3.80(s, 3H, p-OCH 3 ), 3.18 (2H, NH 2 ), 1.61(d, 3H, CHCH 3 ).
[0056] Mass spectrum EI-MS (m / z): 228.5 [M] + .
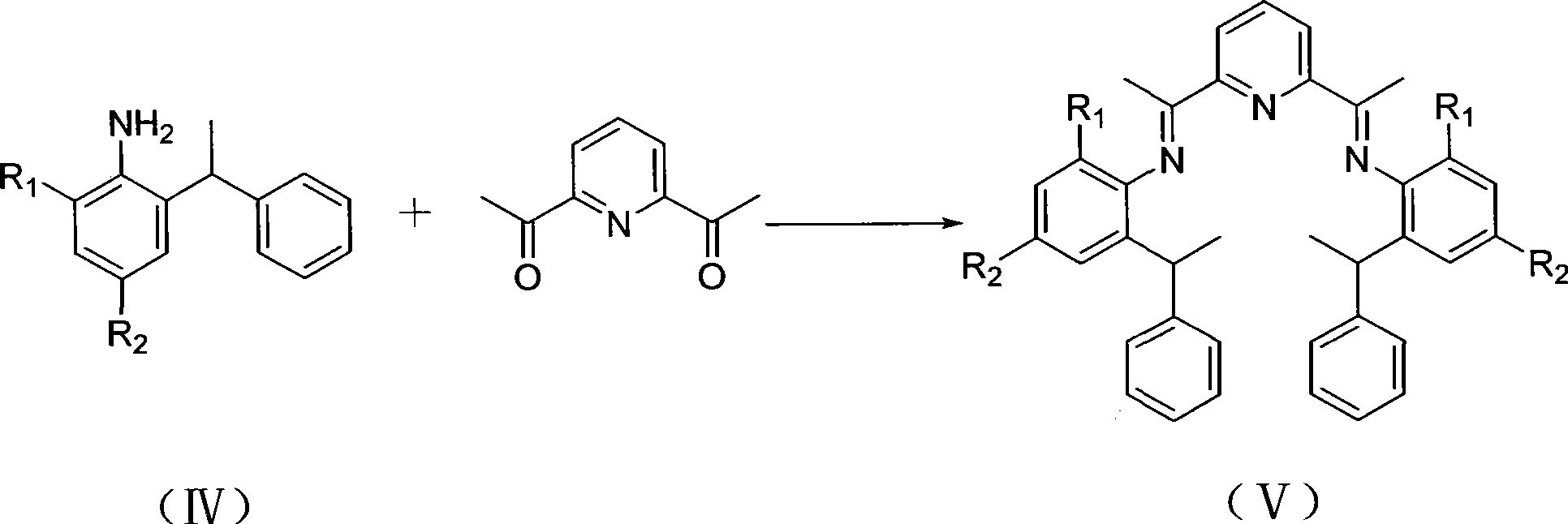
[0057] 2. Pr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com