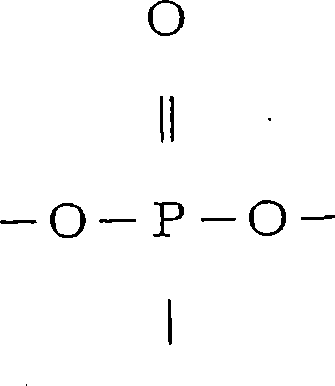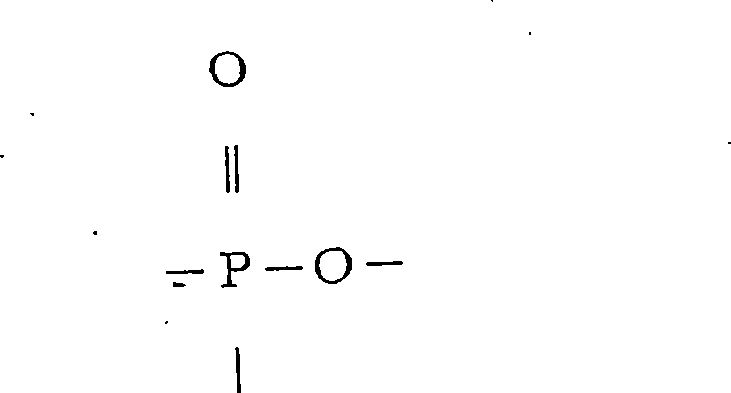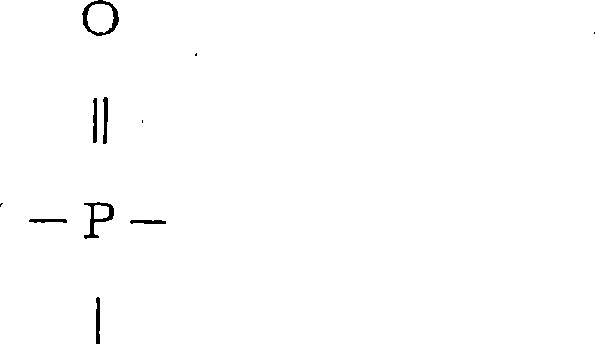Catalyst for polymerization of polyester, polyester and process for preparing polyester
A technology of polymerization catalyst and polyester, which is used in the preparation of polyester and polyester, and the field of polyester polymerization catalyst, can solve the problems of cap pollution, limited addition method, black ink impurities generated by PET, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0359] The polyester of the present invention can be produced by conventionally known methods. For example, when producing PET, polycondensation after esterification between terephthalic acid and ethylene glycol can be used, and alkyl esters of terephthalic acid such as dimethyl terephthalate and the like can also be used. Polymerization after transesterification between ethylene glycol. In addition, the polymerization apparatus may be a batch type or a continuous type.
[0360] The catalysts used in the polymerization of polyesters according to the process according to the invention are catalytically active not only in polycondensation but also in esterification and transesterification. The transesterification reaction between alkyl esters of dicarboxylic acids such as dimethyl terephthalate and diols such as ethylene glycol is usually carried out in the presence of a transesterification catalyst such as zinc, but these catalysts or The catalyst of the present invention is ...
Embodiment 1-1
[0440] (Synthesis example of phosphorus compound)
[0441] Synthesis of a phosphorus compound (phosphorus compound A) represented by the following formula (44)
[0442] (44)
[0443]
[0444] Synthesis of Sodium (O—ethyl3, 5-di—tert—butyl—4—hydroxybenzylphosphonate)
[0445] Add 5 g (14 mmol) of diethyl (3,5-di-tert-butyl-4-hydroxybenzyl) phosphonate (Irganox 1222 (manufactured by CHIBA SPECIALTY CHEMICALS)) to a mixed solution of 6.5 g (84 mmol) of 50% aqueous sodium hydroxide solution and 6.1 ml of methanol ) of methanol solution 6.1ml, heated to reflux under nitrogen atmosphere for 24 hours. After the reaction, add concentrated hydrochloric acid 7.33g (70mmol) while cooling the reaction mixture, filter the precipitate, clean with isopropanol, and distill the filtrate under reduced pressure. Gained The residue was dissolved in hot isopropanol, and the insoluble matter was collected by filtration. After the isopropanol was distilled off under reduced pressure, the residu...
Embodiment 1-2~1-3、 comparative example 1-1
[0459] Polymerization of polyester was carried out in the same manner as in Example 1-1 except that the catalyst was changed. The compounds used as catalysts in each of Examples and Comparative Examples are shown in Table 1, respectively. These compounds were added so that the contents of each metal atom and phosphorus atom in the finally obtained polymer were as shown in Table 1. Table 1 shows the polymerization results of each of the Examples and Comparative Examples, the filter pressure increase during spinning, and the yarn breakage evaluation results during drawing. Irganox 1425 was produced by CHIBA SPECIALTY CHEMICALS, and phosphorus compound A was prepared by the same method as in Example 1-1.
[0460] As shown in the Examples and Comparative Examples, if the metal content in the polyester is within the range claimed in the present invention, the spinning and drawing operability is excellent. On the contrary, if the metal content in the polyester is within the range c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com