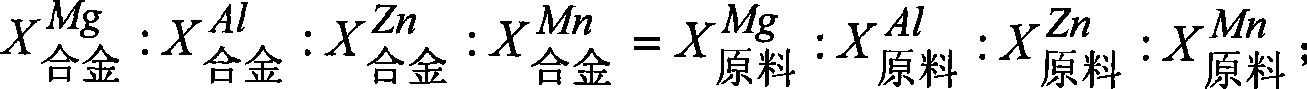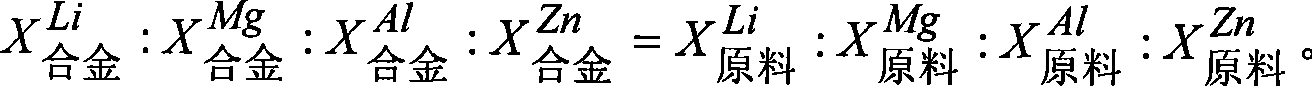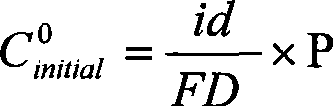Preparation of reactive metal based alloy
A technology of active metal and base alloy, applied in the field of alloy preparation with relatively active metal as the matrix, can solve the problems of no proposed composition control, inability to carry out accurate composition control, difficulty, etc., to shorten the alloy production process, the process is simple and easy, Good uniformity of alloy composition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Taking the preparation of Mg-Al (10wt%) binary alloy as an example, set the following process conditions: direct current: 2A; external heating to make the electrolyte temperature reach 750 ° C; cathode current density 1A / cm 2 ; The initial composition of the electrolyte: magnesium chloride 10wt%-aluminum chloride 0.1wt%-lithium chloride 60wt%-sodium chloride (500g). Under the conditions of the above process parameters, add magnesium chloride-aluminum chloride pre-melted salt, feed once every 30 minutes, after continuous electrolysis for 15 hours, the total amount of feed is 10 times higher than the raw materials of the alloy components other than the main alloy components before electrolysis, The alloy was taken out, and the aluminum content was analyzed by plasma-coupled emission spectrometry, and the current efficiency was calculated and evaluated.
[0023] According to the requirements of the aforementioned feeding:
[0024] (1) The mass ratio of Mg to Al in the fee...
Embodiment 2
[0039] Taking the preparation of Li-Al (80wt%) binary alloy as an example, set the following process conditions: direct current: 2A; electrolyte temperature 650 ° C; cathode current density 1A / cm 2 ; The initial composition of the electrolyte: lithium chloride 40wt% - potassium chloride 60wt% - aluminum chloride 0.1wt%. Under the conditions of the above process parameters, add lithium chloride-aluminum chloride pre-melted salt, feed once every 30 minutes, after continuous electrolysis for 15 hours, the total amount of feed is 10% higher than the raw materials of alloy components other than the main alloy components before electrolysis times, the alloy was taken out, the aluminum content was analyzed by plasma-coupled luminescence spectroscopy, and the current efficiency was calculated and evaluated.
[0040] According to the requirements of the aforementioned feeding:
[0041] (1) The mass ratio of Li and Al in the feed is 1:4, converted into lithium chloride: the mass of alu...
Embodiment 3
[0052] Taking the preparation of Mg-Al (5wt%) binary alloy as an example, set the following process conditions: direct current: 4A; electrolyte temperature 950 ° C; cathode current density 1A / cm 2 ; The initial composition of the electrolyte: magnesium fluoride 20wt% - lithium fluoride 60wt% - sodium fluoride 20wt% - aluminum fluoride 0.1wt%. Under the conditions of the above process parameters, add magnesium oxide and alumina, and feed once every 10 minutes. After continuous electrolysis for 10 hours, the total amount of feed is 10 times higher than the raw materials of the alloy components other than the main alloy components before electrolysis. Take out the alloy and use plasma Coupling luminescence spectroscopic analysis of aluminum content, calculation and evaluation of current efficiency.
[0053] According to the requirements of the aforementioned feeding:
[0054] (1) The mass ratio of Mg to Al in the feed is 19:1, which is 16.67:1 when converted to magnesia:alumina....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com