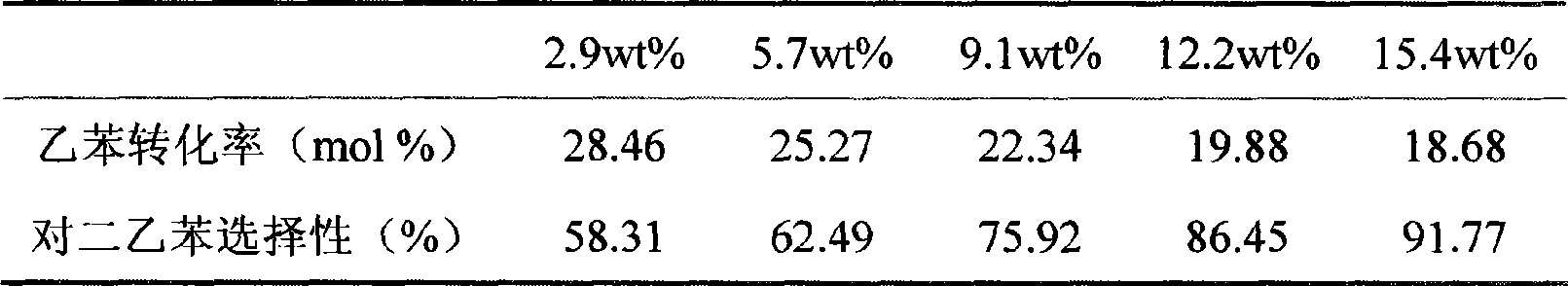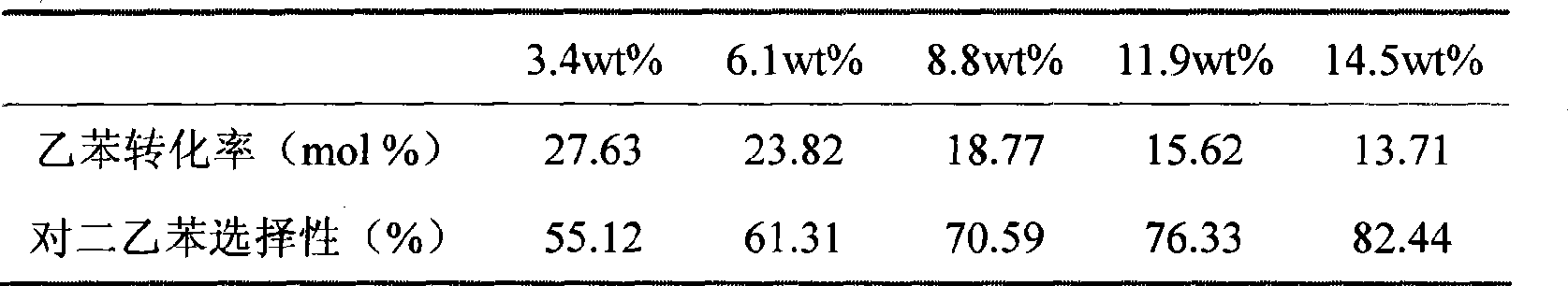Shape-selective catalyst preparation method
A technology for catalysts and shape-selective molecular sieves, which is applied in molecular sieve catalysts, chemical instruments and methods, physical/chemical process catalysts, etc., and can solve problems such as expensive modifiers, heterogeneous catalyst performance, and difficulty in achieving diethylbenzene selectivity. , to achieve good activity and stability, long one-way reaction cycle, and high catalyst selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Prepare hydrogen type HZSM-5 molecular sieve catalyst (molecular sieve grain size is 1-2 micron, SiO 2 / Al 2 o 3 =50, extruded, particle size , molecular sieve content 80%, aluminum oxide content 20%), and it is subjected to water vapor passivation (using 100% water vapor, passivation temperature 550 ℃, passivation time 3 hours, water vapor flux is 1 grams (hours) -1 (g catalyst) -1 ). Then carry out hot impregnation with a concentration of 6wt.% lanthanum nitrate solution (calculated as lanthanum oxide) at 80°C, the impregnation time is 2 hours, and a small amount of dilute nitric acid is added dropwise before the impregnating solution is heated to adjust the pH value to between 3-4 , the amount of rare earth nitrate solution is 5ml (gram catalyst) -1 , the times of hot dipping are 1, 2, 3, 4, 5 times respectively. Drying and roasting are carried out after each impregnation. Drying is carried out at 110°C, and the drying time is 3 hours; calcination is carried...
Embodiment 2
[0064] Prepare hydrogen type HZSM-5 molecular sieve catalyst (molecular sieve grain size is 1-2 micron, SiO 2 / Al 2 o 3 =50, extruded, particle size , molecular sieve content 80%, alumina content 20%), and it is subjected to water vapor passivation treatment (using 100% water vapor, passivation temperature 550 ℃, passivation time 3 hours, water vapor flux is 1 grams (hours) -1 (g catalyst) -1 ). Then carry out hot impregnation with a concentration of 6wt.% lanthanum nitrate solution (calculated as lanthanum oxide) at 80°C, the impregnation time is 2 hours, and a small amount of dilute nitric acid is added dropwise before the impregnating solution is heated to adjust the pH value to between 3-4 , the amount of rare earth nitrate solution is 5ml (gram catalyst) -1 , the number of times of hot dipping is 2 times, and drying and roasting are carried out after each dipping. Drying is carried out at 110°C, and the drying time is 3 hours; calcination is carried out at 550°C, ...
Embodiment 3
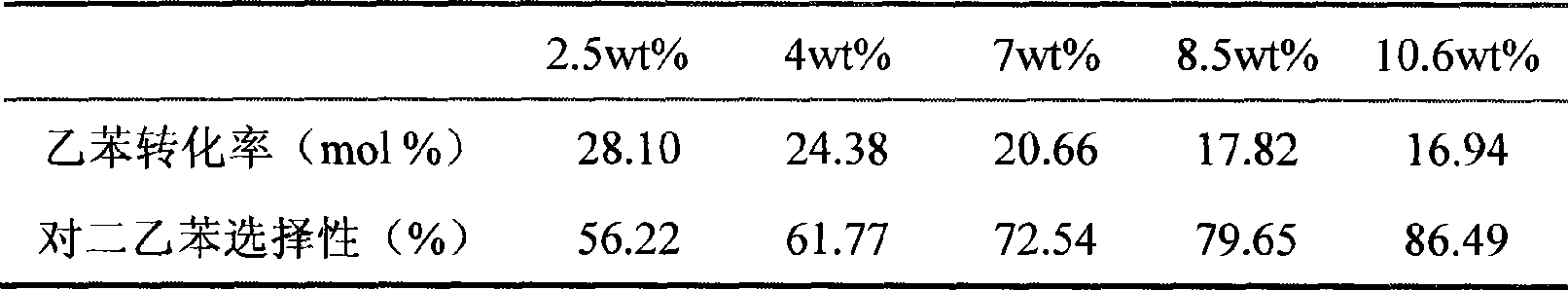
[0066]Repeat Example 2, but use lanthanum-rich mixed rare earth nitrate (lanthanum oxide / rare earth oxide ≥ 40%) dipping solution instead during hot dipping and cold dipping. Then the total loading capacity of the rare earth oxides of the obtained modified catalyst reaches 15.6wt%. The initial reaction performance of the catalyst in the alkylation reaction of ethylbenzene and ethanol is 21.66% of ethylbenzene conversion rate and 96.64% selectivity to diethylbenzene; 22.77%, 96.32% selectivity to diethylbenzene.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com