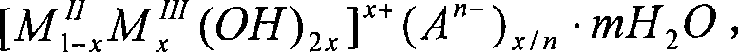Method for removing perchlorate in drinking water
A perchlorate and drinking water technology, applied in chemical instruments and methods, water/sewage treatment, water/sludge/sewage treatment, etc., can solve the problems of small adsorption capacity of activated carbon, difficult analysis of ion exchange, high cost, etc. Achieve the effect of low solubility and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
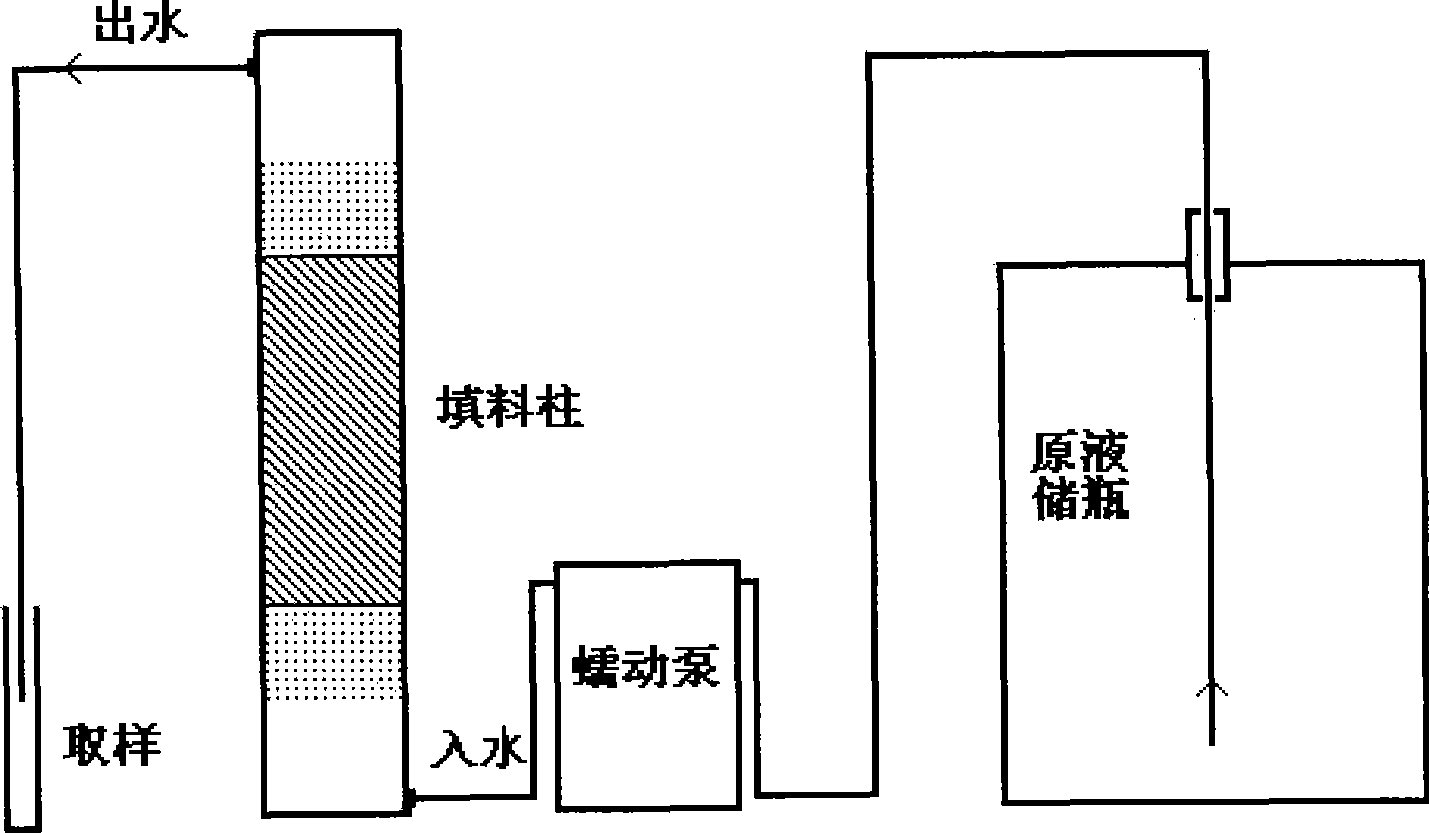
[0015] Example 1: Weigh 47.5 g of magnesium chloride, 33.3 g of aluminum chloride, and 60 g of sodium hydroxide, and dissolve them in 1.5 L of water. The mixed solution was magnetically stirred for 48 hours and then filtered. The solid material was dried at 105° C., and then ground. Take 20g of the prepared material and fill it into a cylindrical packed column, pass the drinking water containing 10-100μg / L of perchlorate through the bottom of the column, and pass through the packed column at a flow rate of 60-100ml / h. The top of the packed column flows out. The results showed that after 100 L of drinking water containing perchlorate was treated, the removal rate of perchlorate reached 93-97%, and its concentration fell below 1 μg / L.
Embodiment 2
[0016] Example 2: Weigh 55.5g of calcium chloride, 40.6g of ferric chloride, and 60g of sodium hydroxide, and dissolve them in 1.5L of water. The mixed solution is magnetically stirred for 48 hours and then filtered. The solid material is dried at 105°C, and then processed Grind. Take 10g of the prepared material and put it into 100L drinking water containing 100-1000μg / L of perchlorate. The mixture was stirred for 1 hour and then filtered. The results showed that after 100L of drinking water containing perchlorate was treated, the removal rate of perchlorate reached 87%, and its concentration fell below 10μg / L.
[0017] The solid material referred to in Example 1 is the inorganic layered compound Mg-Al-LDH (LDH means containing OH - The interlayer compound, the original text of LDH is Layered Double Hydroxides).
[0018] The solid material referred to in Example 2 is the inorganic layered compound Ca-Fe-LDH.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com