Lovastatin and niacin sustained-release preparation and preparation method thereof
A technology of lovastatin and sustained-release preparations, which is applied in pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc. Due to the complex production process and high requirements for storage conditions, the effects of reducing fluctuations, lowering LDL by 45%, and overcoming the need for multiple doses can be achieved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
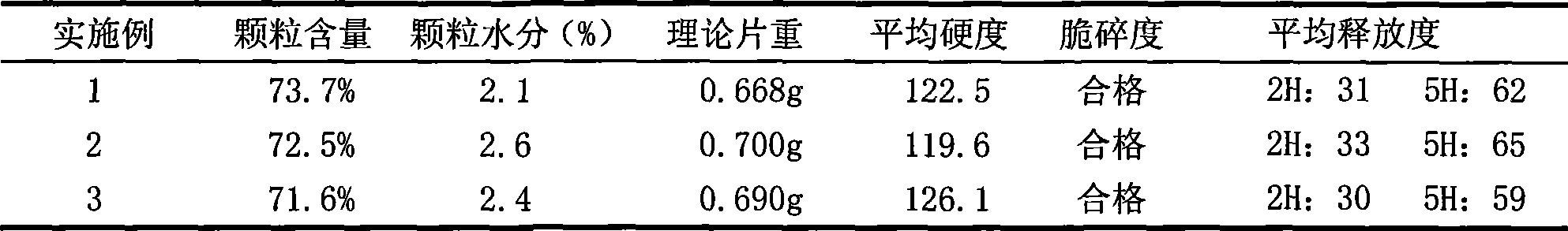
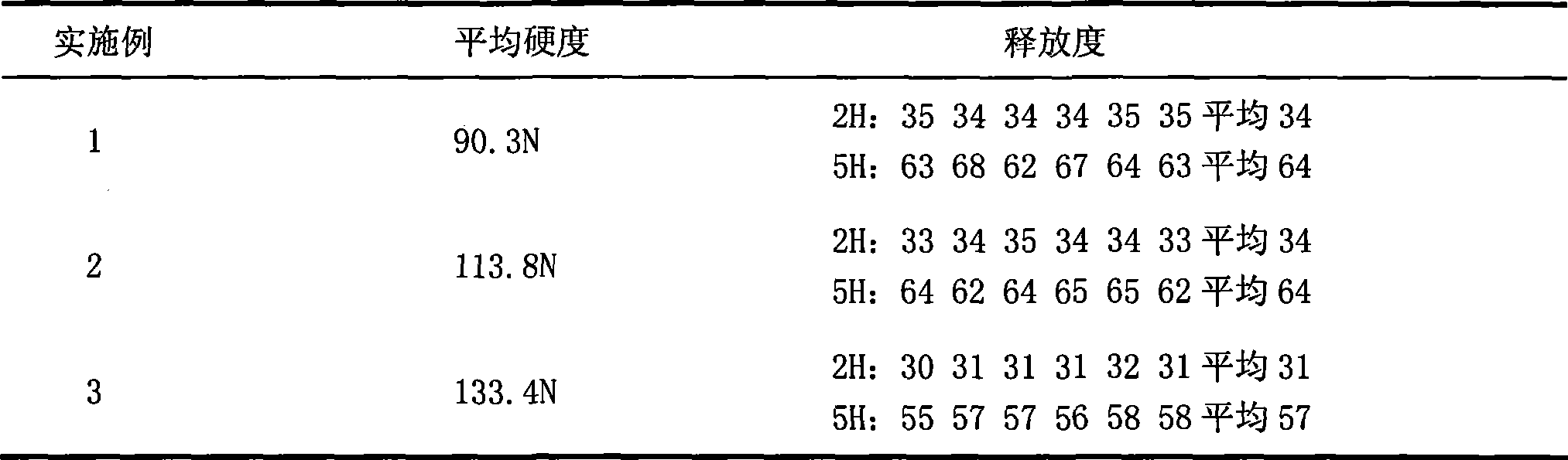
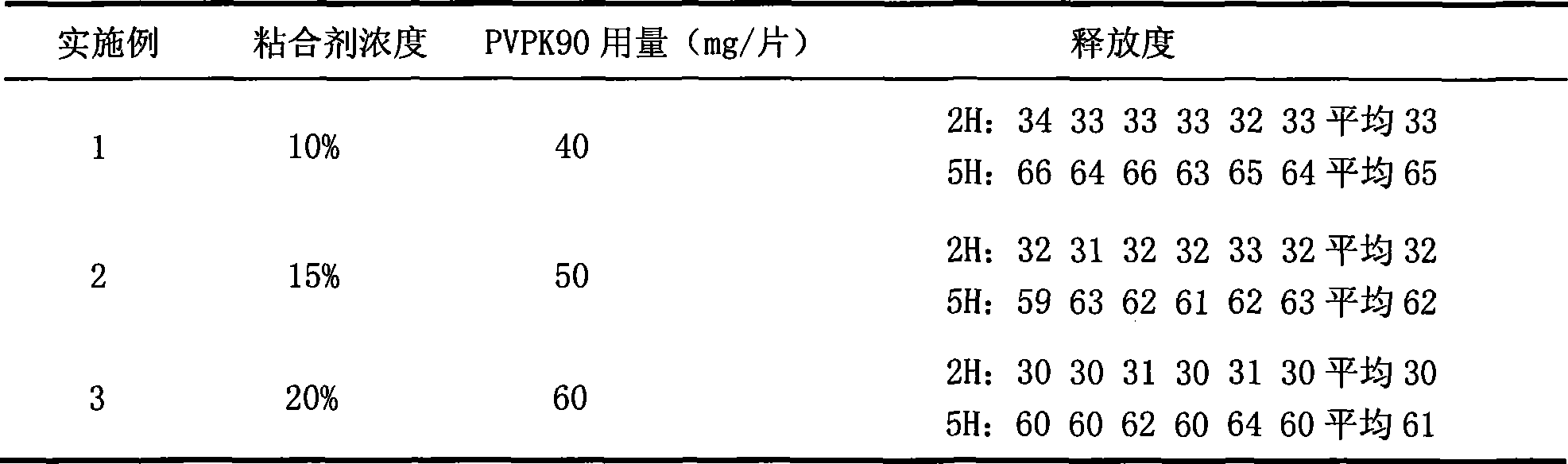
[0027] After the niacin raw material passes through a 100-mesh sieve, according to the weight ratio of 500mg of niacin per tablet, it is fully mixed with 120mg of HPMC K100M skeleton material, and then 50mg of PVPK90 adhesive is added to make a soft material by hand, and wet through a 20-mesh sieve The granules were dried in an oven at 60°C for 2 hours, sieved through a 20-mesh sieve, weighed, added with 6.5 mg of magnesium stearate, and mixed well to make niacin sustained-release layer granules;
[0028] Take lovastatin, pass through a 100-mesh sieve, and mix thoroughly with carboxymethyl starch sodium 5 mg, microcrystalline cellulose 25 mg, starch 15 mg, and lactose 15 mg according to the weight ratio of 20 mg lovastatin per tablet, and add PVPK30 ethanol solution to make it manually For soft materials, pass through a 22-mesh sieve for granulation, place in an oven at 60°C for 1 hour, and granulate the dry granules with a 22-mesh sieve, add 1 mg of magnesium stearate, and mix...
Embodiment 2
[0031] After the niacin raw material passes through a 100-mesh sieve, according to the weight ratio of 500mg of niacin per tablet, it is fully mixed with 130mg of HPMC K100M skeleton material, and then 50mg of PVPK90 adhesive is added to make a soft material by hand, and wet through a 20-mesh sieve The granules were dried in an oven at 60°C for 2 hours, sieved through a 20-mesh sieve, weighed, added with 6.5 mg of magnesium stearate, and mixed well to make niacin sustained-release layer granules;
[0032] Take lovastatin, pass through a 100-mesh sieve, and mix thoroughly with carboxymethyl starch sodium 5 mg, microcrystalline cellulose 25 mg, starch 15 mg, and lactose 15 mg according to the weight ratio of 20 mg lovastatin per tablet, and add PVPK30 ethanol solution to make it manually For soft materials, pass through a 22-mesh sieve for granulation, place in an oven at 60°C for 1 hour, and granulate the dry granules with a 22-mesh sieve, add 1 mg of magnesium stearate, and mix...
Embodiment 3
[0034] After the niacin raw material passes through a 100-mesh sieve, according to the weight ratio of 500mg of niacin per tablet, it is fully mixed with 140mg of HPMC K100M skeleton material, and then 50mg of PVPK90 ethanol solution is added to make a soft material by hand, and wet granules are made through a 20-mesh sieve , placed in an oven and dried at 45°C for 2 hours, granulated through a 20-mesh sieve, weighed, added with 6.5 mg of magnesium stearate, and mixed well to make niacin sustained-release layer granules;
[0035]Take lovastatin, pass through a 100-mesh sieve, and mix thoroughly with carboxymethyl starch sodium 5 mg, microcrystalline cellulose 25 mg, starch 15 mg, and lactose 15 mg according to the weight ratio of 20 mg lovastatin per tablet, and add PVPK30 ethanol solution to make it manually The soft material is granulated through a 22-mesh sieve, dried in an oven at 60°C for 1 hour, the dry granules are granulated through a 22-mesh sieve, and 1 mg of magnesiu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com