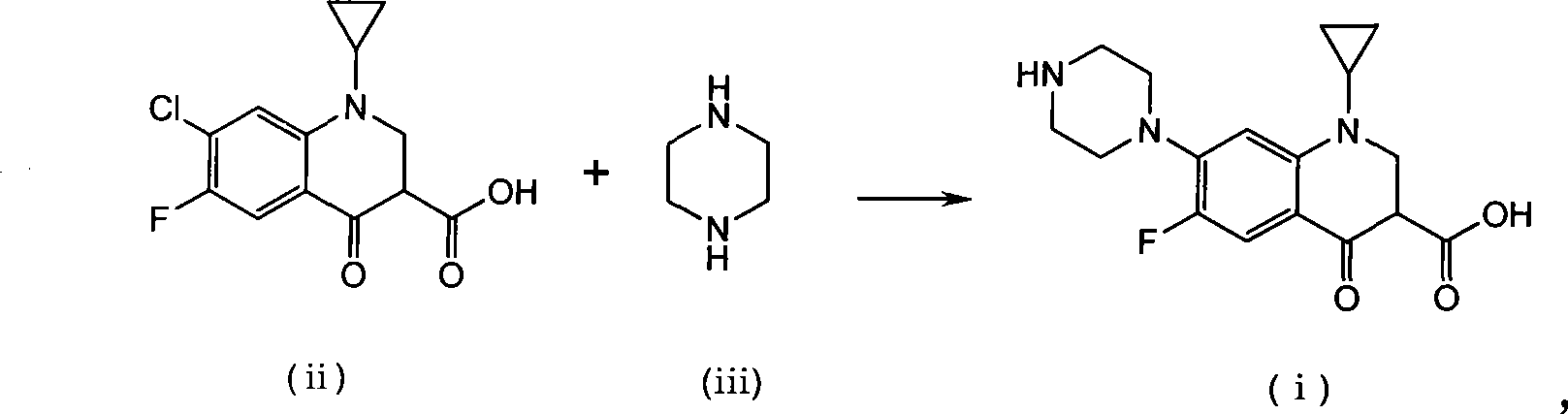
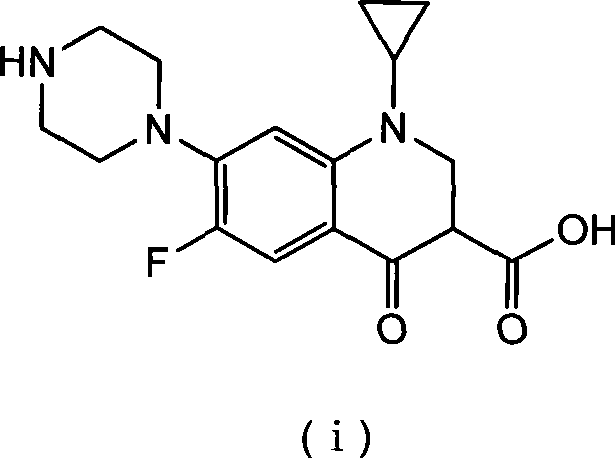
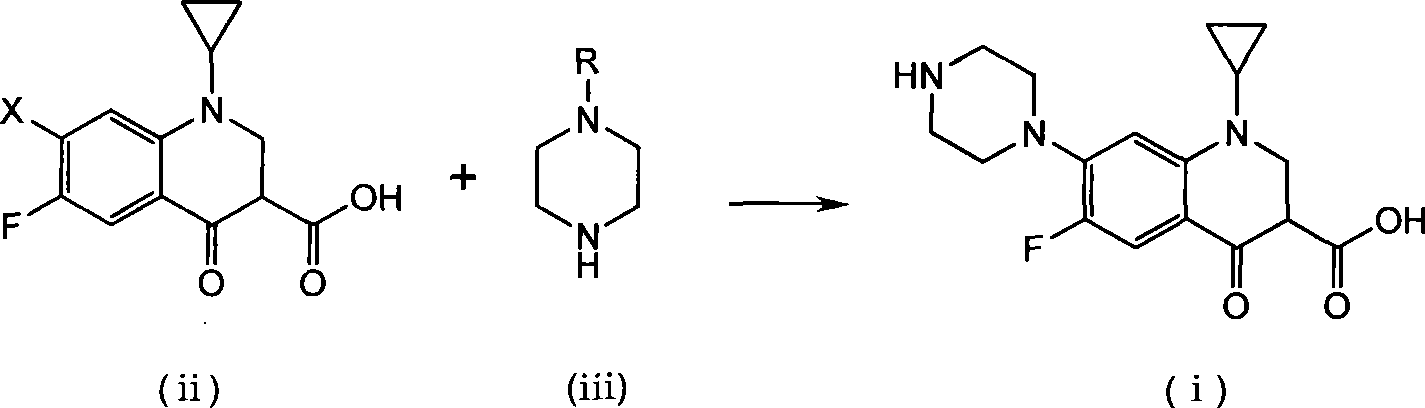
Method for preparing ciprofloxacin by piperazine reaction
A technology of ciprofloxacin and piperazine, which is applied in the fields of organic chemistry and antibacterial drugs, can solve the problems such as the yield needs to be improved and the reaction time is long, so as to shorten the production cycle, improve the reaction efficiency and reduce environmental pollution Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] a) Put 100.0g of cyclopropanecarboxylic acid and 141.2g of hexapentapiperazine into a 1L four-necked bottle, heat slowly to 70-80°C, add 3g of catalyst anhydrous aluminum trichloride, heat to about 120°C, and reflux for 1~ 3h, TLC monitors that the reaction is complete. b) Add additive Na 2 HPO 4 15g, add 18.5g / 50ml of NaOH / water solution dropwise, adjust the pH value of the reaction system to 9~12; after the dropwise addition, continue to stir for 20min, add 3g of activated carbon, reflux for 0.5h for decolorization, and filter while hot (keep the filtration temperature above 80°C ). c) Adjust the pH value of the filtrate to 7-8 with hydrochloric acid, stir and crystallize at room temperature, raise the temperature to 70-80°C, stir for 0.5h, filter while hot, wash the filter cake with 150ml of hot purified water at about 50°C for 3 times; bake the filter cake After drying, the crude product of ciprofloxacin was obtained as light yellow solid, about 116g. The crude...
Embodiment 2
[0038] a) Put 100.0g of cyclopropanecarboxylic acid and 61.2g of anhydrous piperazine into a 1L four-necked bottle, slowly heat to 70-80°C, add 5g of catalyst crystallized aluminum trichloride, 100g of purified water, heat to about 115°C, and reflux The reaction was carried out for 3-4 hours, and the reaction was monitored by TLC to complete. b) Add 6g of potassium benzoate as an auxiliary agent, add 18.5g / 50ml of NaOH / water solution dropwise, adjust the pH value of the reaction system to 9-12; after the dropwise addition, continue to stir for 20min, add 3g of activated carbon, reflux for 0.5h for decolorization, and heat Filter (keep the filter temperature above 80°C). c) Adjust the pH value of the filtrate to 7-8 with hydrochloric acid, stir and crystallize at room temperature, raise the temperature to 70-80°C, stir for 0.5h, filter while hot, wash the filter cake with 150ml of hot purified water at about 50°C for 3 times; bake the filter cake After drying, the crude produc...
Embodiment 3
[0042] a) Put 100.0g of cyclopropanecarboxylic acid and 188.3g of hexapentapiperazine into a 1L four-neck bottle, heat slowly to 70-80°C, add 6g of catalyst aluminum sulfate, heat to about 120°C, reflux for 1-3h, monitor by TLC The reaction went to completion. b) Add 18.5g / 50ml of NaOH / water solution dropwise to adjust the pH value of the reaction system to 9-12; after the dropwise addition, continue to stir for 20min, add 3g of activated carbon, reflux for 0.5h for decolorization, and filter while it is hot (keep the filtration temperature above 80°C ). c) Adjust the pH value of the filtrate to 7-8 with hydrochloric acid, stir and crystallize at room temperature, raise the temperature to 70-80°C, stir for 0.5h, filter while hot, wash the filter cake with 150ml of hot purified water at about 50°C for 3 times; bake the filter cake After drying, the crude product of ciprofloxacin was obtained as light yellow solid, about 116g. The crude yield is 99%.
[0043] HPLC analysis an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com