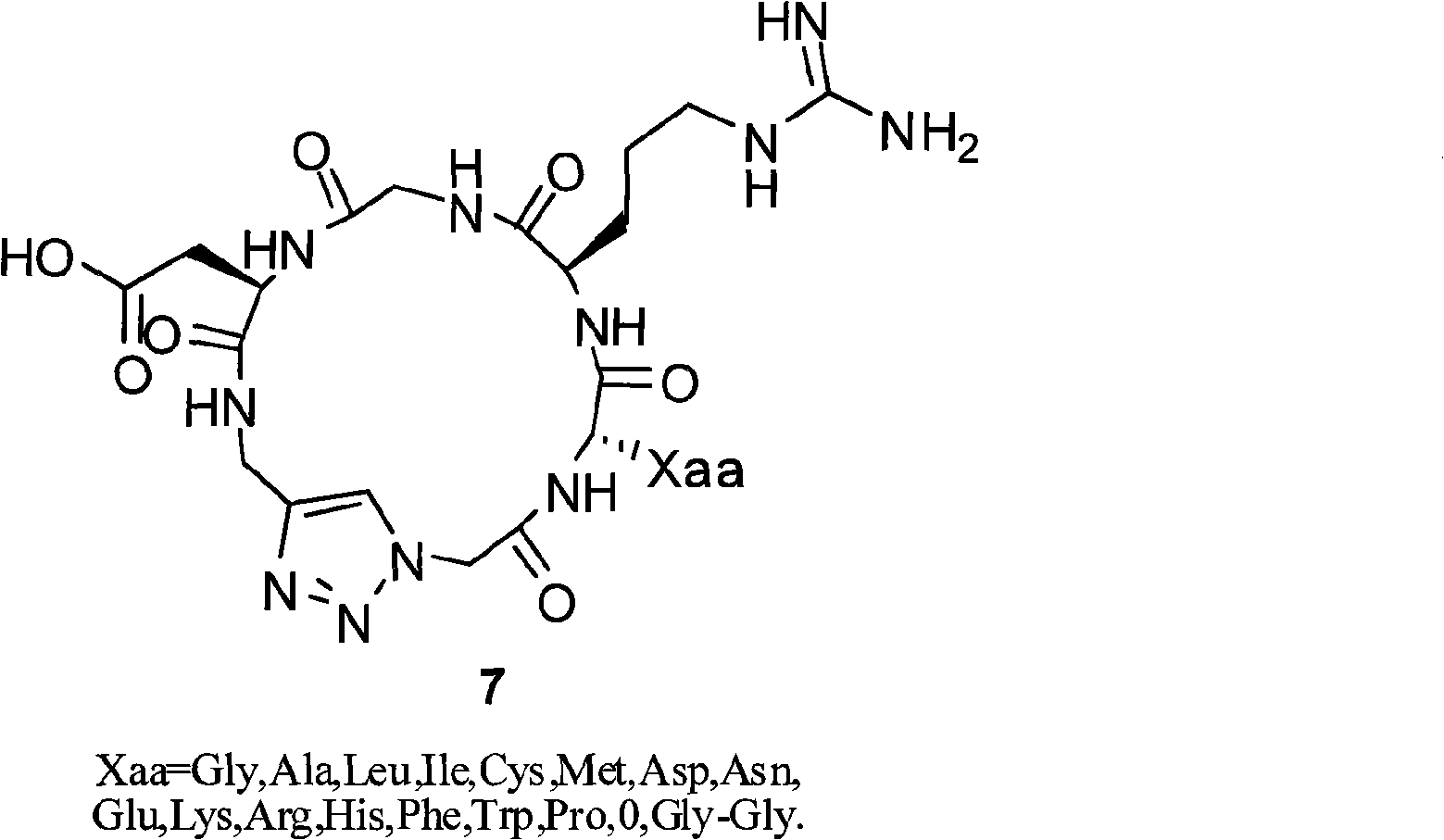
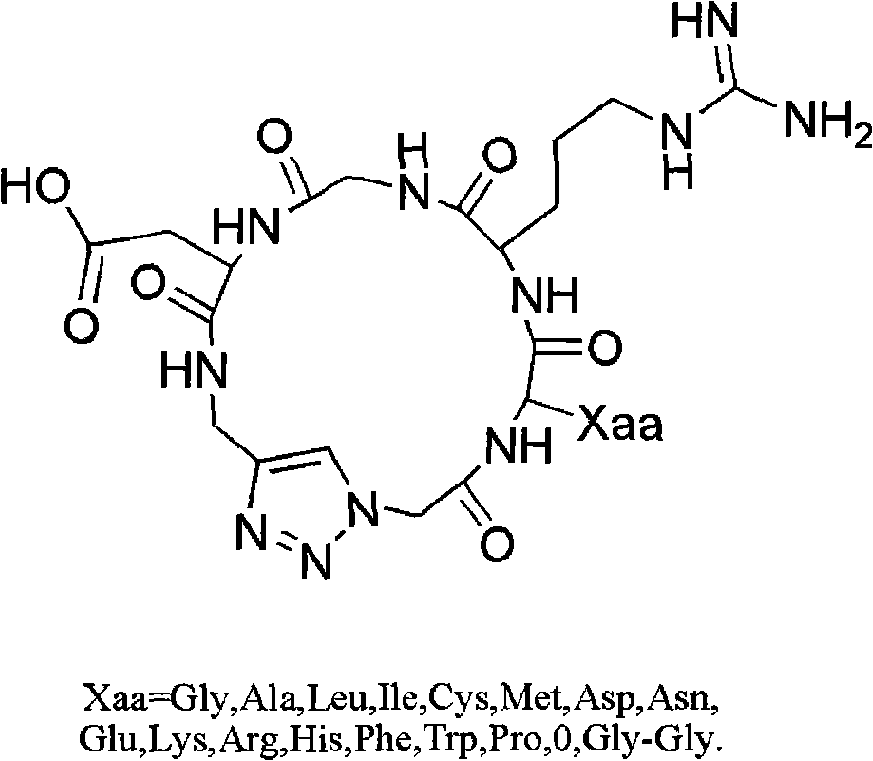
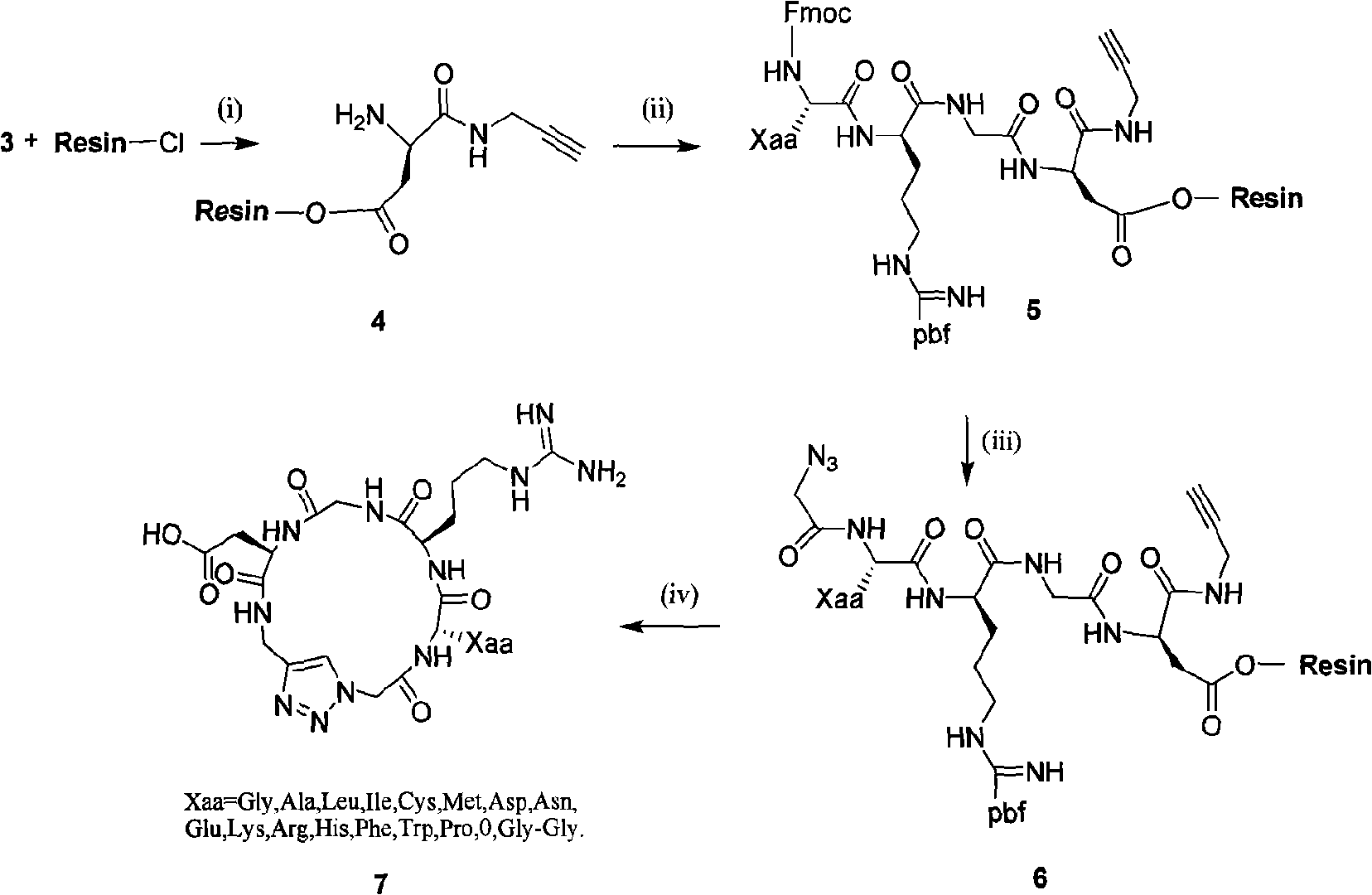
Integrin ligand cyclic peptide analogues and cyclization method
A technology of analogs and integrins, applied in the field of cyclic peptide analogs and their cyclization, can solve the problems of reduced cyclization yield, etc., and achieve the effects of mild conditions, increased biological activity, and simple cyclization method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] 1.1 Preparation of trifluoromethanesulfonyl azide:
[0041] Dissolve sodium azide (6 g) in a 150 ml round bottom flask, add 24 ml of DCM, cool to 0° C. in an ice bath, and stir for 20 minutes. The reaction mixture was extracted with DCM (2×12ml). The organic phases were combined and washed with saturated sodium carbonate to obtain trifluoromethanesulfonyl azide dissolved in DCM, which was directly used in the next reaction without further purification.
[0042] 1.2 Preparation of azidoglycine:
[0043] Glycine (0.675g), K 2 CO 3 (1.783g), CuSO 4 (0.0226g) was added to a round bottom flask, then water (30ml), methanol (60ml) and DCM (48ml) containing trifluoromethanesulfonyl azide were added, stirred at room temperature, and reacted overnight. Subsequently, the organic solvent was removed by rotary evaporation under reduced pressure, and the remaining part was diluted with water (150ml). Then the pH value was adjusted to 6 with hydrochloric acid, diluted with phospha...
Embodiment 1
[0053] 1. Synthesis of Cyclo[-Arg-Gly-Asp-ψ(triazole)-Gly-Gly-](7a):
[0054] 1. Synthesis of Azido-glycine (according to the principle of diazonium transfer):
[0055] 1.1 Preparation of trifluoromethanesulfonyl azide:
[0056] Dissolve sodium azide (6 g) in a 150 ml round bottom flask, add 24 ml of DCM, cool to 0° C. in an ice bath, and stir for 20 minutes. The reaction mixture was extracted with DCM (2×12ml). The organic phases were combined and washed with saturated sodium carbonate to obtain trifluoromethanesulfonyl azide dissolved in dichloromethane, which was directly added to the next reaction without further purification.
[0057] 1.2 Preparation of azidoglycine:
[0058] Glycine (0.675g), K 2 CO 3 (1.783g), CuSO 4 (0.0226g) was added to a round bottom flask, then water (30ml), methanol (60ml) and dichloromethane (48ml) containing trifluoromethanesulfonyl azide were added, stirred at room temperature, and reacted overnight. Subsequently, the organic solvent was ...
Embodiment 2
[0069] Synthesis of Cyclo[-Arg-Gly-Asp-ψ(triazole)-Gly-Ala-](7b):
[0070] The steps in this embodiment are the same as in Embodiment 1, wherein:
[0071] In the third step, Fmoc-Arg(Pbf)-OH, fluorenylmethoxycarbonyl-alanine (Fmoc-Ala-OH) and Azido-glycine are sequentially condensed to generate linear tetrapeptide analogs.
[0072] The physicochemical analysis data of gained final product are as follows:
[0073] Cyclo[-Arg-Gly-Asp-ψ(triazole)-Gly-Ala-](7b).White solids, mp: 164-165℃; [ α ] D 25 = + 56.3 (c=1.0in CHCl 3 ); 1 H NMR (500MHz, DMSO-d 6 ): δ1.23 (1H, J = 6.3Hz, d), 1.28 (2H, J = 7.0Hz, d), 1.38-1.45 (2H, m), 1.87-1.93 (2H, m), 2.67-2.75 ( 2H, m), 2.89(2H, s), 4.12-4.19(3H, m), 4.21(1H, J=5.0Hz, d), 4.28(1H, J=6.3Hz, d), 4.39(1H, J =14.3, 7.2Hz, dd), 4.40(1H, J=7.1Hz, d), 5.07(1H, J=6.3Hz, d), 5.27(1H, J=16.6Hz, d), 6.92(2H, s ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com