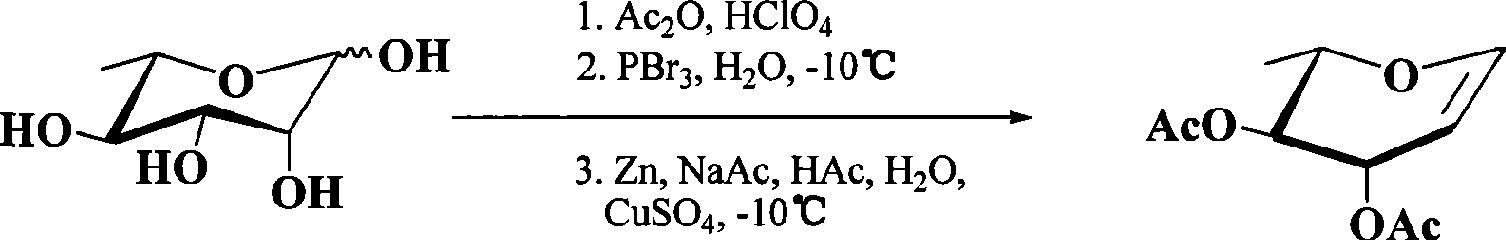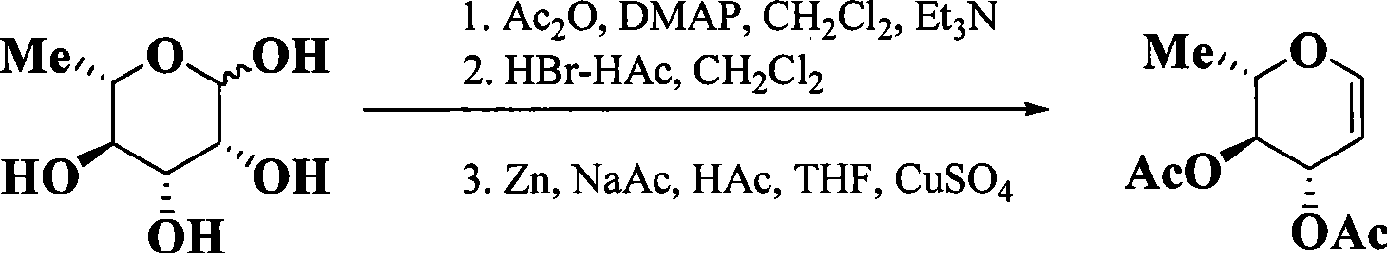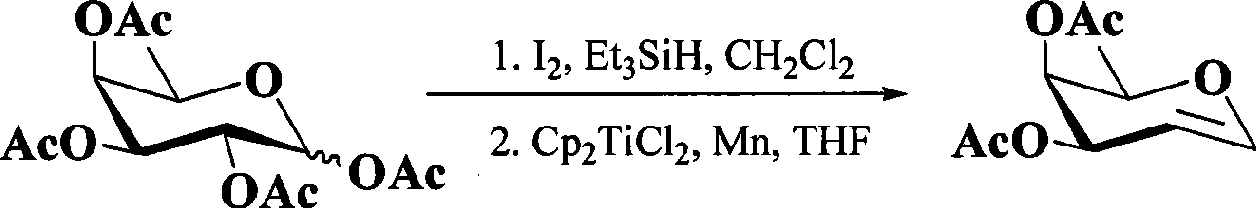Preparation of 3,4-di-O-acetyl-L-rhamnal
A technology of rhamnetose and acetyl group, applied in 3 fields, can solve the problems of high cost, complicated intermediate product processing and the like, and achieve the effect of reducing cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0019] Example 1: Add 1.0g rhamnose, 5.0mL acetic acid, 3.5mL acetic anhydride, and 20mg p-toluenesulfonic acid to a 50mL round bottom flask, stir and react at room temperature for 10 minutes, then add 1.25 acetyl bromide to the reaction solution mL, methanol 0.8mL, and react in the dark for 1 hour. The reaction solution was extracted with dichloromethane, the organic phase was washed 3 times with water, 2 times with saturated aqueous sodium bicarbonate solution, and 1 time with saturated aqueous sodium chloride solution, then dried by adding anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure to obtain 1- Bromo-2,3,4-tri-O-acetyl-L-rhamnose crude.
[0020] Dissolve the generated 1-bromo-2,3,4-tri-O-acetyl-L-rhamnose in 5 mL of ethyl acetate, then add 20 mL of saturated sodium dihydrogen phosphate aqueous solution and 4 g of zinc powder, and react for 1 hour. Filtrate, extract the filtrate 3 times with ethyl acetate, combine the organic phases, wash...
example 2
[0021] Example 2: Add 1.0g rhamnose, 10mL acetic acid, 3.5mL acetic anhydride, 20mg catalytic amount of perchloric acid to a 50mL round bottom flask, stir and react at room temperature for 30 minutes, then add 1.25mL acetyl bromide to the above reaction solution , methanol 0.8mL, and react in the dark for 2 hours. The reaction solution was extracted with dichloromethane, the organic phase was washed 3 times with water, 2 times with saturated aqueous sodium bicarbonate solution, 1 time with saturated aqueous sodium chloride solution, dried by adding anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure to obtain 1-bromo - Crude 2,3,4-tri-O-acetyl-L-rhamnose.
[0022] Dissolve the resulting 1-bromo-2,3,4-tri-O-acetyl-L-rhamnose in 10 mL of ethyl acetate, add 20 mL of phosphate buffer (made from 4.4 g of disodium hydrogen phosphate, 5.6 g of phosphoric acid Prepared by sodium dihydrogen and 20mL water) and 4g zinc powder, reacted at room temperature for ...
example 3
[0023] Example 3: Add 1.0g rhamnose, 7mL acetic acid, 3.5mL acetic anhydride, 20mg catalytic amount of zinc chloride to a 50mL round bottom flask, stir and react at room temperature for 1.5 hours, then add 1.25mL acetyl bromide to the above reaction solution , methanol 0.8mL, and react in the dark for 3 hours. The reaction solution was extracted with dichloromethane, the organic phase was washed 3 times with water, 2 times with saturated aqueous sodium bicarbonate solution, and 1 time with saturated aqueous sodium chloride solution, dried by adding anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure to obtain 1-bromo - Crude 2,3,4-tri-O-acetyl-L-rhamnose.
[0024] Dissolve the resulting crude 1-bromo-2,3,4-tri-O-acetyl-L-rhamnose in 8 mL of acetone, then add 20 mL of saturated potassium dihydrogen phosphate aqueous solution and 4 g of zinc powder, and react for 5 hours. Filtrate, extract the filtrate 3 times with ethyl acetate, combine the organic p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com