Preparation method of tralkoxydim
A technology of triclotrione and trimethylbenzaldehyde, applied in the field of preparation of triclotrione, can solve the problems of low product yield, layered emulsification, complicated steps of triclotrione, etc., and achieves high product quality, The effect of increasing the reaction speed and avoiding by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
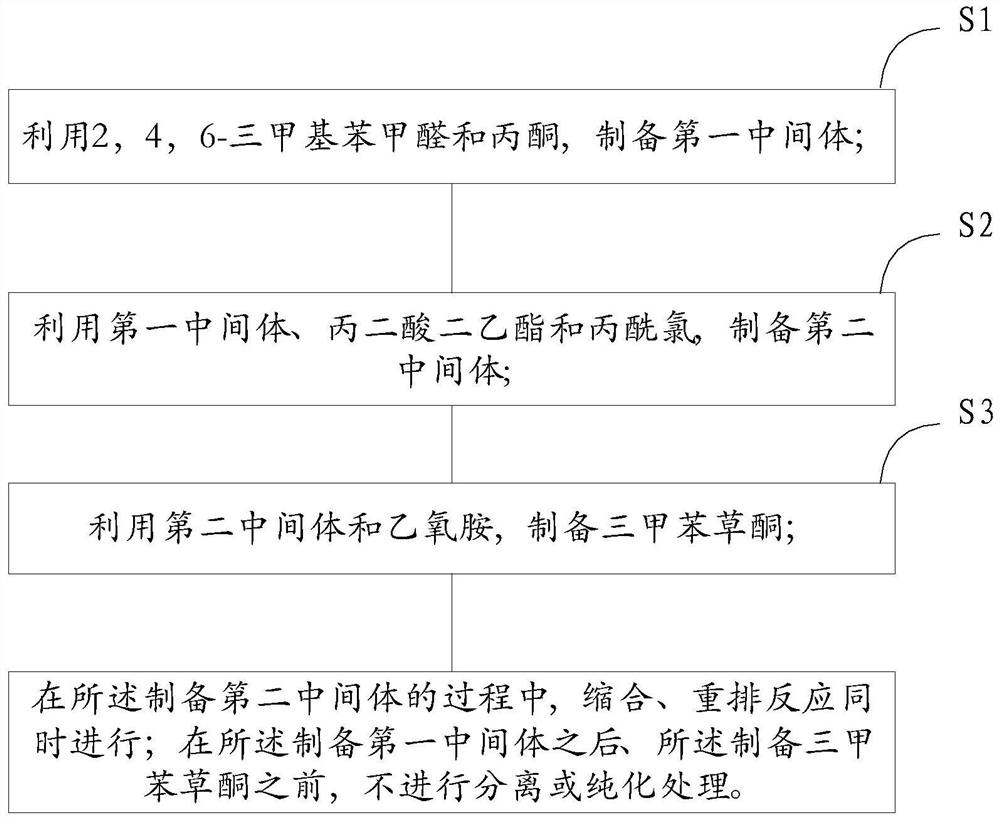
[0049] figure 1 It is a process flow chart of the method for preparing trimethylbenzotrione according to the embodiment of the present invention. The preparation method of the embodiment of the present invention can be applied to the preparation of trimethylbenzotrione, and the preparation method is figure 1 each step in the .
[0050] figure 1 A kind of method for preparing trimethylbenzotrione provided by the present invention is shown, and described method comprises:
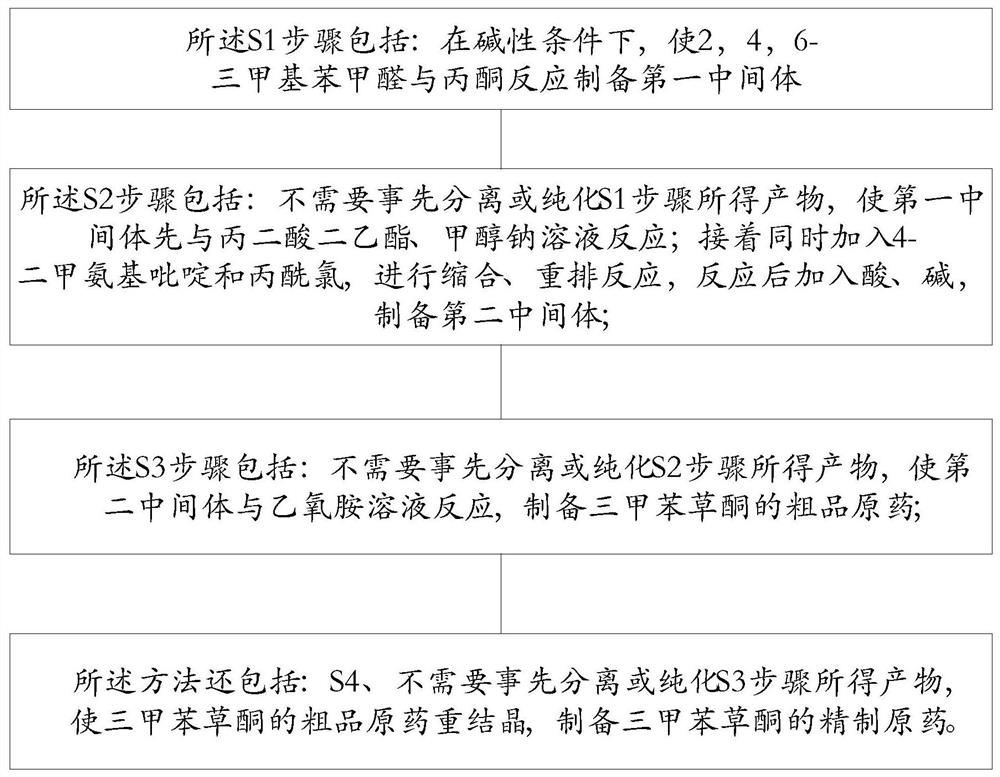
[0051] S1, using 2,4,6-trimethylbenzaldehyde and acetone to prepare the first intermediate;
[0052] S2, using the first intermediate, diethyl malonate and propionyl chloride to prepare the second intermediate;
[0053] S3, using the second intermediate and ethoxyamine to prepare trimethylbenzotrione;
[0054] In the process of preparing the second intermediate, condensation and rearrangement reactions are carried out simultaneously; after the preparation of the first intermediate and before the preparat...
example 1
[0088] This example adopts a one-pot method to prepare trimethylbenzotrione. It should be noted that the product obtained in the previous step does not need to be separated or purified in advance between the following steps.
[0089] 1. Synthesis of the first intermediate
[0090] The synthetic route of the first intermediate is:
[0091]
[0092] first intermediate
[0093] Table 1 first intermediate synthesis feed list
[0094]
[0095] Steps:
[0096] Put 2,4,6-trimethylbenzaldehyde, acetone, water, and sodium hydroxide solution into the four-necked bottle according to the actual dosage shown in Table 1, heat up to reflux under stirring, and keep the temperature within the range of 72-76°C Keep reflux for 4 hours; remove about 90mL of solvent under normal pressure, then cool down to below 70°C, add toluene, separate layers, reflux the toluene layer and divide water, then cool down to 25-30°C to obtain the toluene solution of the first intermediate.
[0097] 2. Syn...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com