Preparation method of selective estrogen receptor degradation agent and intermediate thereof
A technology of selecting and reducing agents, applied in organic chemistry and other fields, can solve the problems of complex post-processing and low atom utilization rate, and achieve the effect of simple post-processing, high atom utilization rate, and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
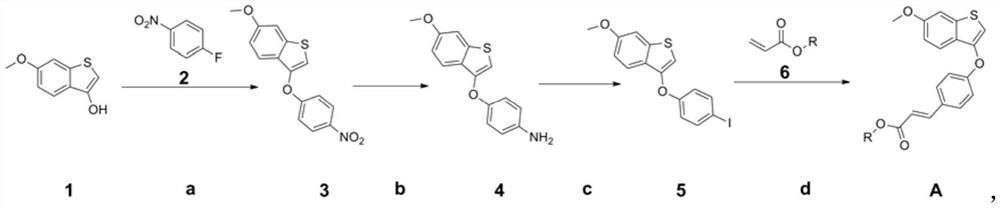
[0105] (E)-tert-butyl 3-(4-((6-methoxybenzo[b]thiophen-3-yl)oxy)phenyl)acrylate (Intermediate A 1 ) preparation
[0106]
[0107] Intermediate A of this example 1 The preparation route is as shown above, and preparation method comprises the steps:
[0108]a. Dissolve 60g of compound 1 in 1L N,N-dimethylacetamide, cool in ice water to about 0°C, slowly add 14.6g of sodium hydrogen in batches, remove the ice-water bath, react at about 30°C for 30min, drop Add 51.7g of compound 2 and react at 30°C for 2h. Pour the reaction solution into 400mL of 1M hydrochloric acid aqueous solution, extract 3 times with ethyl acetate, combine the organic phases, wash 3 times with water in turn, wash with saturated aqueous sodium chloride solution, dry over anhydrous sodium sulfate, filter, concentrate, and column chromatography to obtain pure product Compound 3 (83 g, 83%).
[0109] b. Dissolve 65g of Compound 3 in a mixed solvent of 240mL of water and 1.2L of ethanol, add 60g of iron pow...
Embodiment 2
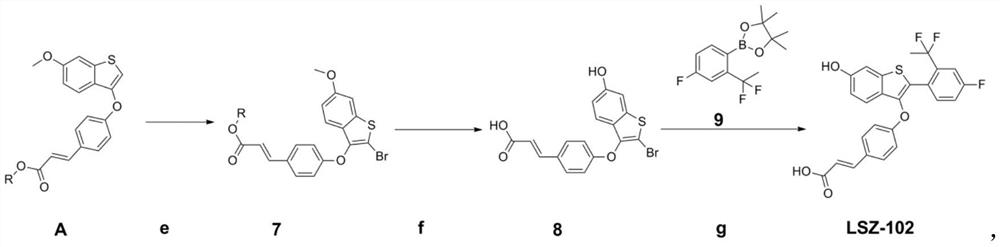
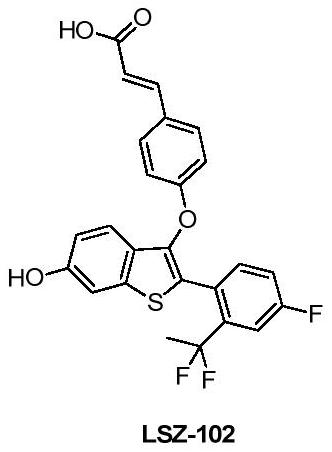
[0118] (E)-3-(4-((2-(2-(1,1-difluoroethyl)-4-fluorophenyl)-6-hydroxybenzo[b]thiophen-3-yl)oxy ) phenyl) the preparation method of acrylic acid (LSZ-102)
[0119]
[0120] The preparation route of LSZ-102 (SER degradation agent) of the present embodiment is as shown above, and preparation method comprises the following steps:
[0121] e, the 28g compound A 1 Added into 500mL THF, then added 13.6g N-bromosuccinimide, reacted at room temperature for 3h, concentrated to remove THF, and separated by column chromatography to obtain pure compound 7-1 (31g, 94%).
[0122] f. Dissolve 32.9g of compound 7-1 in 350mL of dichloromethane, under argon protection, cool to 0°C, slowly add 156.5mL of 1M boron tribromide in dichloromethane solution dropwise, stir at room temperature for 18h, and pour the reaction solution slowly into 1L saturated aqueous sodium bicarbonate solution, stirred evenly, extracted three times with tertiary methyl ether, combined the organic phases, washed with 1...
Embodiment 3
[0130] (E)-methyl 3-(4-((6-methoxybenzo[b]thiophen-3-yl)oxy)phenyl)acrylate (Intermediate A 2 ) preparation
[0131]
[0132] Intermediate A of this example 2 The preparation route is as shown above, and preparation method comprises the steps:
[0133] d. Dissolve 50g of compound 5 in 1L of N,N-dimethylformamide, add 78.8g of compound 6-2, 65g of triethylamine, and 8.5g of bis(triphenylphosphine)palladium chloride, under argon protection, for 1 liter to 80°C, stirred for 4 hours, filtered to remove inorganic salts and palladium catalysts, diluted the mother liquor with 1.5 L of water, extracted three times with ethyl acetate, combined the organic phases, washed three times with water, washed with saturated sodium chloride, dried over anhydrous sodium sulfate, filtered, Concentrate and separate by column chromatography to obtain pure product LSZ-102 intermediate compound A 2 (37.8g, 85%).
[0134] Compound A 2 : ESI-MS: m / z C 19 h 16 o 4 S[M+H] + Calculated: 341.1; ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com