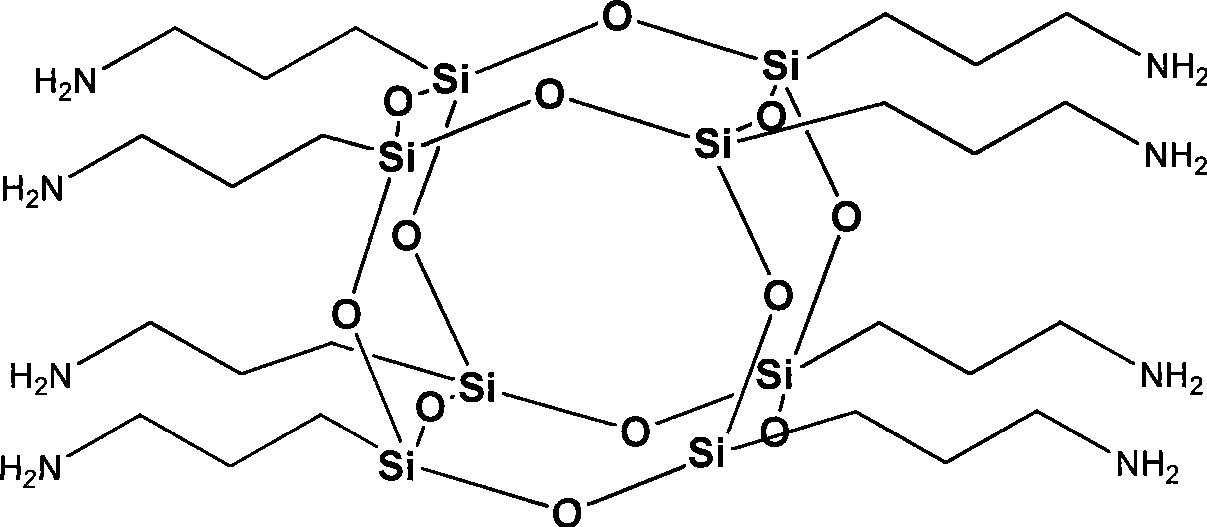
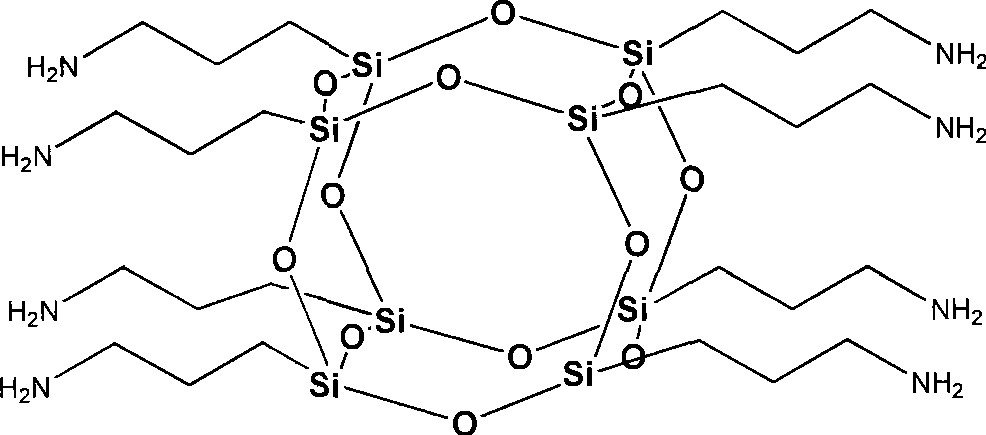
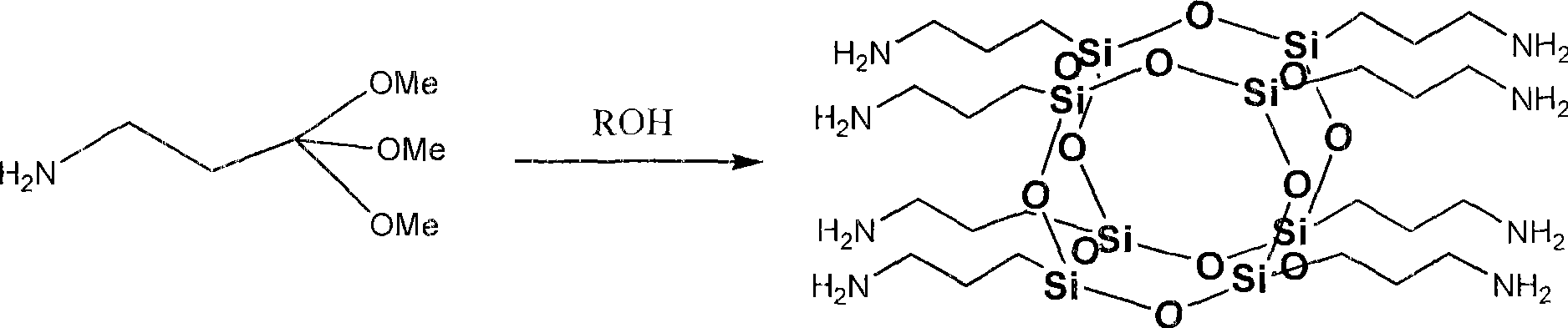
Octa-aminopropyl cage type sesquialter siloxane and preparation thereof
A technology of silsesquioxane and octaaminopropyl cages, which is applied in the direction of silicon organic compounds, can solve the problems of complex formation process, insufficient understanding of formation mechanism, and numerous influencing factors, so as to improve yield and avoid random compounds The formation and the effect of simplifying the preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0024] Add 500ml of ethanol, 50ml of deionized water, and 1.3ml of tetramethylammonium hydroxide into a 1000ml flask in sequence, and stir at room temperature to make the mixture even. Then, 66.39 g of γ-aminopropyltriethoxysilane was added dropwise to the above mixed solution, followed by stirring. After the dropwise addition, the reaction was continued at this temperature for 3h. Then the temperature was raised to 80° C., and the reaction was continued for 10 h under reflux, and the reaction ended. The reaction solution obtained in step (1) was distilled under reduced pressure, concentrated to 80ml, and then the concentrated solution was added dropwise to 1600ml of petroleum ether. After the dropwise addition, it was left to stand for 3 hours, and a large amount of white solids were precipitated. After vacuum filtration, washing the crude product with acetone, and vacuum drying, 29.73 g of octaaminopropyl clathrate silsesquioxane was finally obtained, with a yield of 90%. ...
example 2
[0029] Add 360ml of methanol, 30ml of deionized water, and 1.4ml of tetraethylammonium hydroxide into a 500ml flask, and stir at room temperature to make the mixture even. Then, 35.85 g (0.2 mol) of γ-aminopropyltrimethoxysilane was added dropwise to the above mixed solution, followed by stirring. After the dropwise addition, the reaction was continued at this temperature for 5h. Then the temperature was raised to 70° C., and the reaction was continued for 12 h under reflux, and the reaction was completed. The above reaction solution was distilled under reduced pressure, concentrated to 50ml, and then the concentrated solution was added dropwise to 1000ml of petroleum ether, and left to stand after the dropwise addition until a large amount of white solids were precipitated. After vacuum filtration, washing the crude product with acetone, and vacuum drying, 20.27 g of octaaminopropyl clathrate silsesquioxane was finally obtained, with a yield of 92%. The structural character...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com