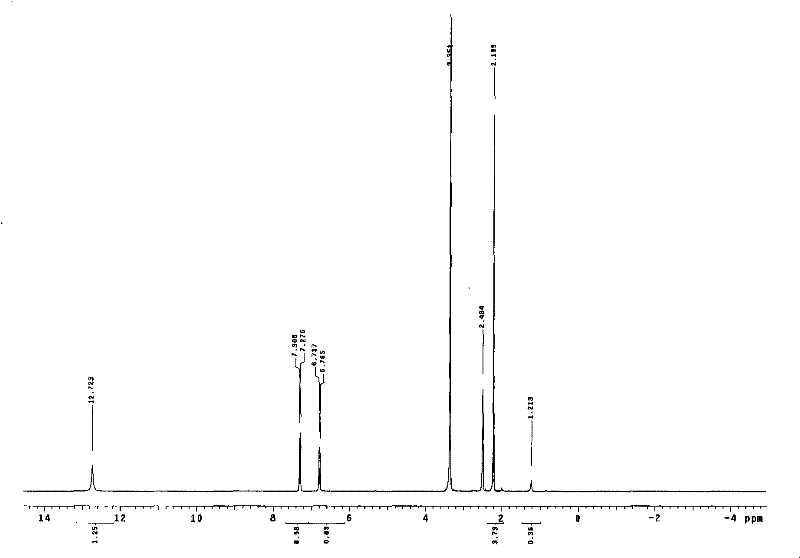
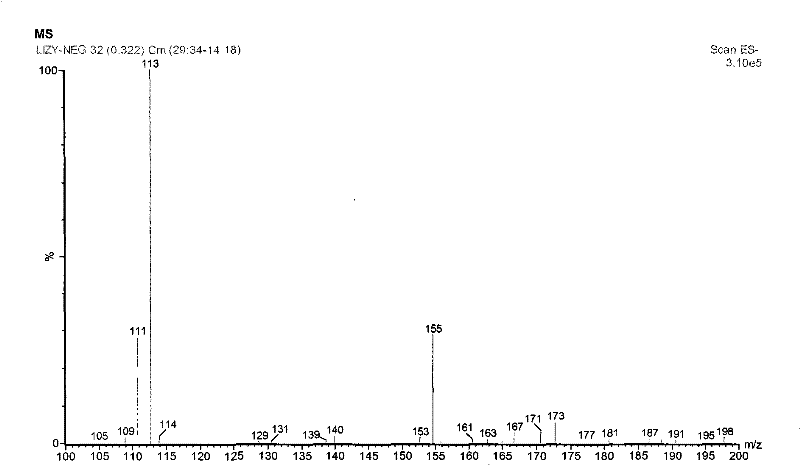
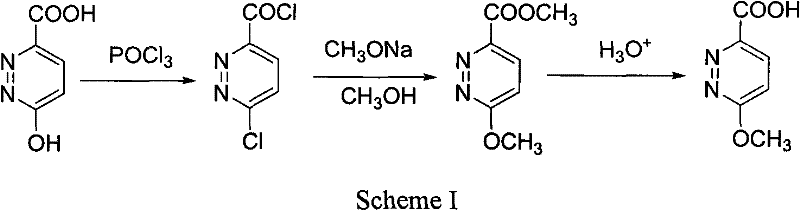
Synthesis process of 6-methoxy pyridazine-3- carboxylic acid
A technology of methoxypyridazine and synthesis process, applied in the direction of organic chemistry, etc., can solve the problems of complex preparation, limited sources, and limitations of 6-hydroxypyridazine-3-carboxylic acid, and shorten the synthesis steps and reduce the operation process. , suitable for the effect of industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Using 3-chloro-6-methylpyridazine as the starting material, the methyl group was oxidized to carboxylic acid to obtain 6-chloropyridazine-3-carboxylic acid, which was methoxylated with chlorine to obtain the target compound with a total yield of 37.7%.
[0020]
[0021] Preparation of 6-chloropyridazine-3-carboxylic acid In ice-cooling, 8 g (0.06 mol) of 3-chloro-6-methylpyridazine was added to 60 ml of concentrated sulfuric acid. Under stirring, 35 g (0.12 mol) of potassium dichromate was added in portions. After reacting at 50° C. for 2 h, add 200 ml of ice water to dilute after cooling, and extract with ethyl acetate 200 ml×5. The extracts were combined, dried over anhydrous sodium sulfate, and reduced to dryness under reduced pressure. The residue was recrystallized from methanol to obtain 6.2 g of white crystalline powder with a yield of 65%.
[0022] Preparation of 6-methoxypyridazine-3-carboxylic acid In 50 ml of anhydrous methanol, 2.2 g (0.04 mol) of sodi...
Embodiment 2
[0025] Using 3-chloro-6-methylpyridazine as the starting material, the methyl group was oxidized to carboxylic acid to obtain 6-chloropyridazine-3-carboxylic acid, which was methoxylated with chlorine to obtain the target compound with a total yield of 30.2%.
[0026]
[0027] Preparation of 6-chloropyridazine-3-carboxylic acid Under ice cooling, 8 g (0.06 mol) of 3-chloro-6-methylpyridazine was added to 60 ml of 50% sulfuric acid. Under stirring, 38 g (0.24 mol) of potassium permanganate was added in portions. After reacting at 80° C. for 2 h, add 200 ml of ice water to dilute after cooling, filter, and extract the filtrate with ethyl acetate 100 ml×4. The extracts were combined, dried over anhydrous sodium sulfate, and reduced to dryness under reduced pressure. The residue was recrystallized from methanol to obtain 5.0 g of white crystalline powder with a yield of 52% and a melting point of 148-151°C.
[0028] Preparation of 6-methoxypyridazine-3-carboxylic acid In 50 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com