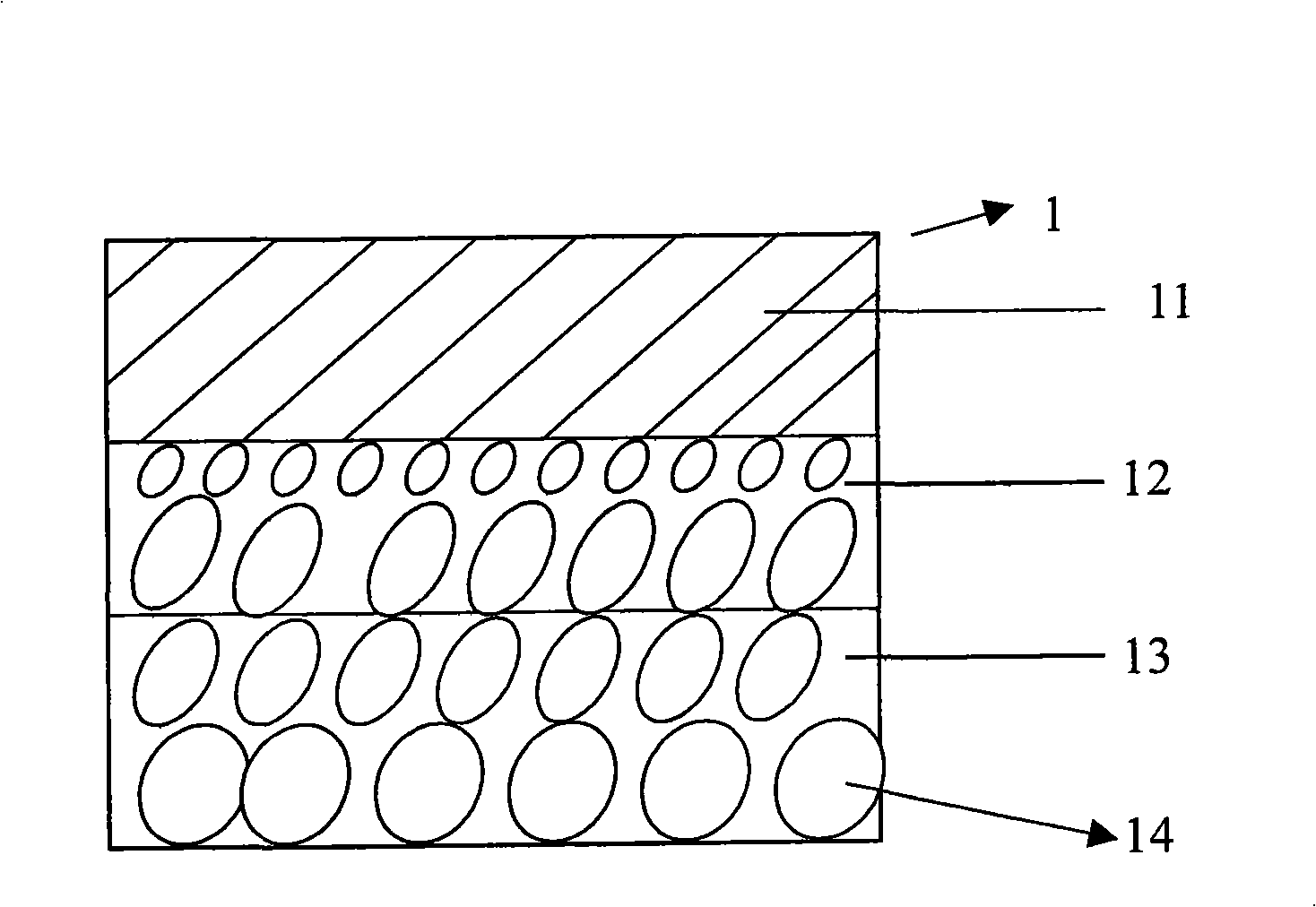
Drug-eluting coronary artery stent and method for preparing same
A coronary artery and drug technology, applied in the medical field of treating cardiovascular diseases, can solve the problems of LAST and stent poor adherence, hinder the vascular endothelialization on the stent surface, poor biocompatibility, etc. Biocompatible properties, uniform release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] 300 mg of drug rapamycin (Rapamycin) (dissolved in acetone to form a 10% rapamycin acetone (Acetone) solution) was injected into 300 ml of chitosan acidic aqueous solution. The percentage by weight of each substance in the solution is 3% of chitosan; 2% of acetic acid; 2% of sodium chloride; and 93% of distilled water. (wherein chitosan is a drug microsphere encapsulation material, aqueous acetic acid is a solvent for chitosan, and sodium chloride plays a role in promoting the dissolution of chitosan better), so that the obtained
[0042] Drug / chitosan=12%-18% solution.
[0043] The chitosan acidic aqueous solution was mixed with 3-5 ml of liquid paraffin / ether mixture containing 0.85 g of emulsifier—Sorbitan Sesquioleate (Sorbitan Sesquioleate, Arlacel 83). This mixed liquor is the solvent of emulsifier, and its component weight ratio is
[0044] Liquid paraffin / ether=35 ml / 25 ml.
[0045] The liquid containing chitosan acidic aqueous solution, emulsifier, and drug ...
Embodiment 2
[0048] Drug rapamycin (Rapamycin) 200 mg (dissolved in acetone to form 1% rapamycin acetone (Acetone) solution) was injected into 250 ml chitosan acidic aqueous solution. The percentage by weight of each substance in the acidic solution is 2% chitosan; 1% acetic acid; 97% distilled water. (wherein chitosan is a drug microsphere encapsulation material, and the aqueous acetic acid solution is a solvent for chitosan), so that obtaining
[0049] Drug / chitosan=13%-17% solution.
[0050] The sodium tripolyphosphate (TPP) aqueous solution whose mass concentration is 0.45 mg-1.0 mg / ml is slowly added dropwise to the above-mentioned drug-containing chitosan acetic acid solution under high-speed stirring at 2000 rpm, and the solution is The mass concentration of the chitosan is 0.5 mg-1.0 mg / ml, and the dropping quantity is that the mass ratio of the chitosan acetic acid solution containing the medicine to the sodium tripolyphosphate solution is 5:1-10:1. Control the pH value (PH valu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com