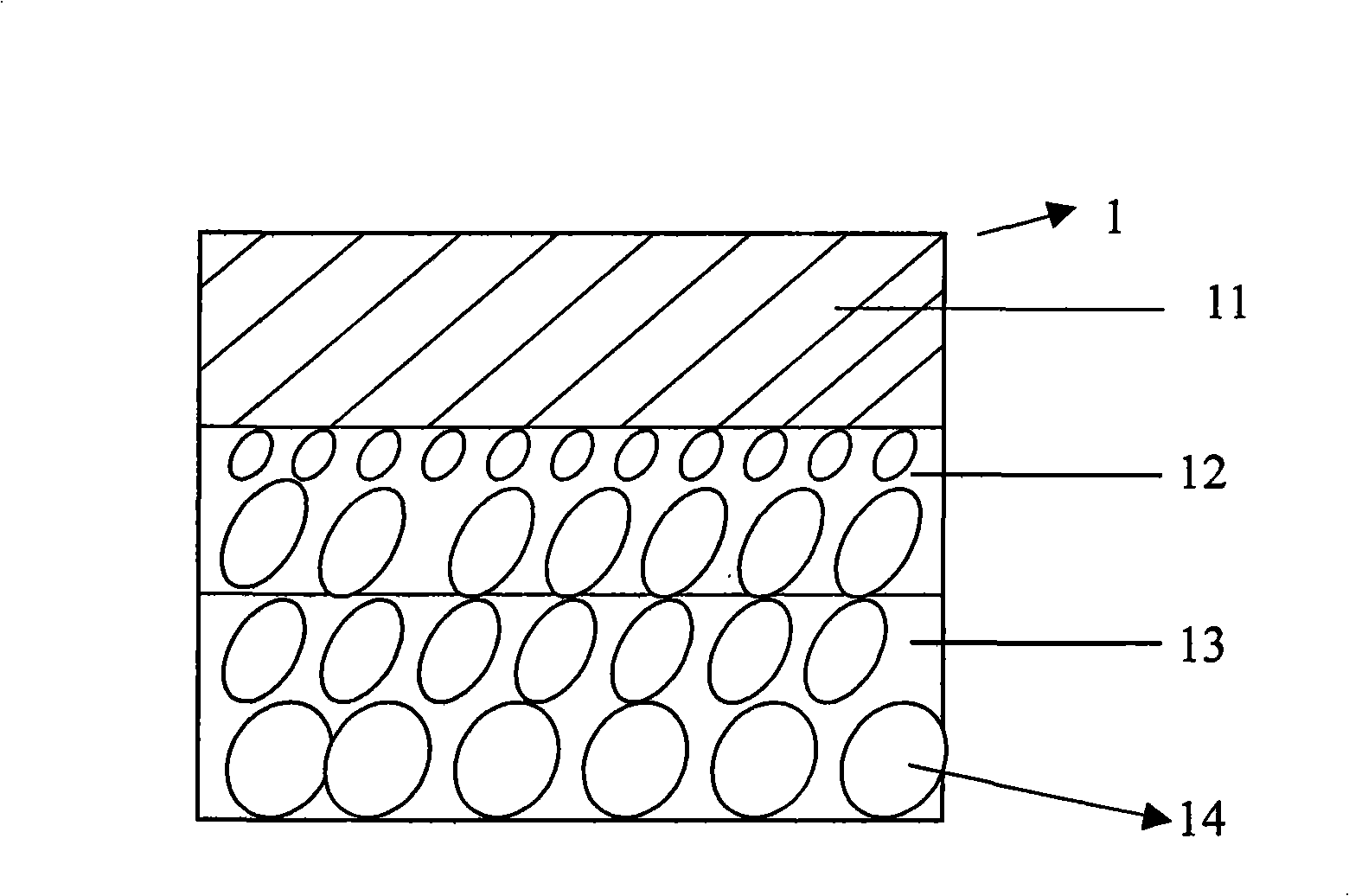
Encapsulation drug microsphere of medicament-eluting coronary artery stent and method for preparing same
A technology of drugs and microspheres, applied in the medical field of treating cardiovascular diseases, can solve the problems of obstructing the vascular endothelialization on the surface of stents, LAST and stent malapposition, high sensitization and inflammatory reaction, and achieve excellent biocompatibility, Reduce the phenomenon of blasting and release, which is beneficial to the effect of crawling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] 300 mg of drug rapamycin (Rapamycin) (dissolved in acetone to form a 10% rapamycin acetone (Acetone) solution) was injected into 300 ml of chitosan acidic aqueous solution. The percentage by weight of each substance in the solution is 3% of chitosan; 2% of acetic acid; 2% of sodium chloride; and 93% of distilled water. (wherein chitosan is a drug microsphere encapsulation material, aqueous acetic acid is a solvent for chitosan, and sodium chloride plays a role in promoting the dissolution of chitosan better), so that the obtained
[0048] medicine / chitosan= A solution (where the A value is between 12%-18%, including two critical values).
[0049] The chitosan acidic aqueous solution was mixed with 3-5 ml of liquid paraffin / ether mixture containing 0.85 g of emulsifier—Sorbitan Sesquioleate (Sorbitan Sesquioleate, Arlacel 83). This mixed liquor is the solvent of emulsifier, and its component weight ratio is
[0050] Liquid paraffin / ether=35 ml / 25 ml.
[0051] The liq...
Embodiment 2
[0056] Drug rapamycin (Rapamycin) 200 mg (dissolved in acetone to form 1% rapamycin acetone (Acetone) solution) was injected into 250 ml chitosan acidic aqueous solution. The percentage by weight of each substance in the acidic solution is 2% chitosan; 1% acetic acid; 97% distilled water. (wherein chitosan is a drug microsphere encapsulation material, and the aqueous acetic acid solution is a solvent for chitosan), so that obtaining
[0057] medicine / chitosan= A solution (A value is between 13% and 17%).
[0058] Set the mass concentration to B(B value is between 0.45 mg-1.0 mg / ml) sodium tripolyphosphate (TPP) aqueous solution is slowly added dropwise to the above-mentioned drug-containing chitosan acetic acid solution under the high-speed stirring of 2000 rpm, and The mass concentration of chitosan in the solution was C (C value is between 0.5 milligram-1.0 milligram / ml), and the quantity of dropping is that the mass ratio of medicine-containing chitosan acetic acid solu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com