Method for synthesizing glycocyamine and salt thereof
The technology of a guanidinoacetate and a synthetic method is applied in the synthesis field of guanidinoacetic acid and its salt, can solve the problems of low yield, complicated reaction process and the like, and achieves convenient separation and purification, simple synthesis process and easy process conditions. control effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1, the preparation of guanidine acetate
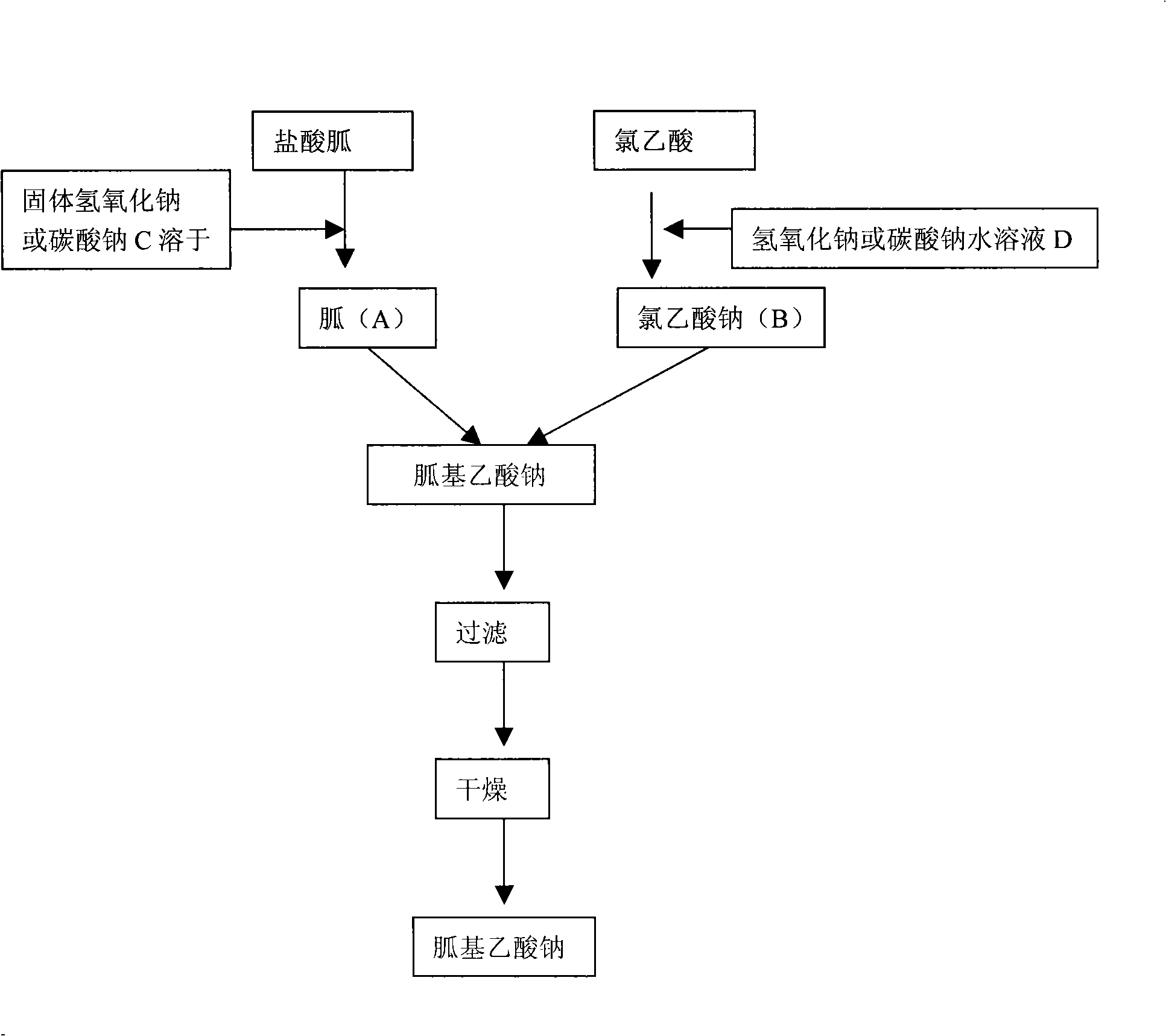
[0044] The present invention prepares the flow process of guanidine acetate as figure 1 Shown:
[0045] 1. Preparation of guanidine acetate
[0046]1. Preparation of free guanidine solution
[0047] Add 200gNaOH (or 265gNaOH) to 600ml water 2 CO 3 ) stirring and dissolving, cooling to below 15°C, slowly adding 480g of guanidine hydrochloride, stirring and dissolving until transparent. The free guanidine solution A can be obtained.
[0048] 2. Sodium chloroacetate solution
[0049] Add 460g chloroacetic acid in 460ml water and stir at room temperature until dissolved, add dropwise 25% (mass percentage) NaOH solution 760g (or 30% (mass percentage) NaOH solution 2 CO 3 solution 840g), stirred until completely dissolved. Sodium chloroacetate solution B can be obtained.
[0050] 3. Synthesis of guanidinoacetate
[0051] The free guanidine solution A prepared in step (1) was added dropwise to the sodium chloroace...
Embodiment 2
[0066] Embodiment 2, the preparation of guanidinoacetic acid
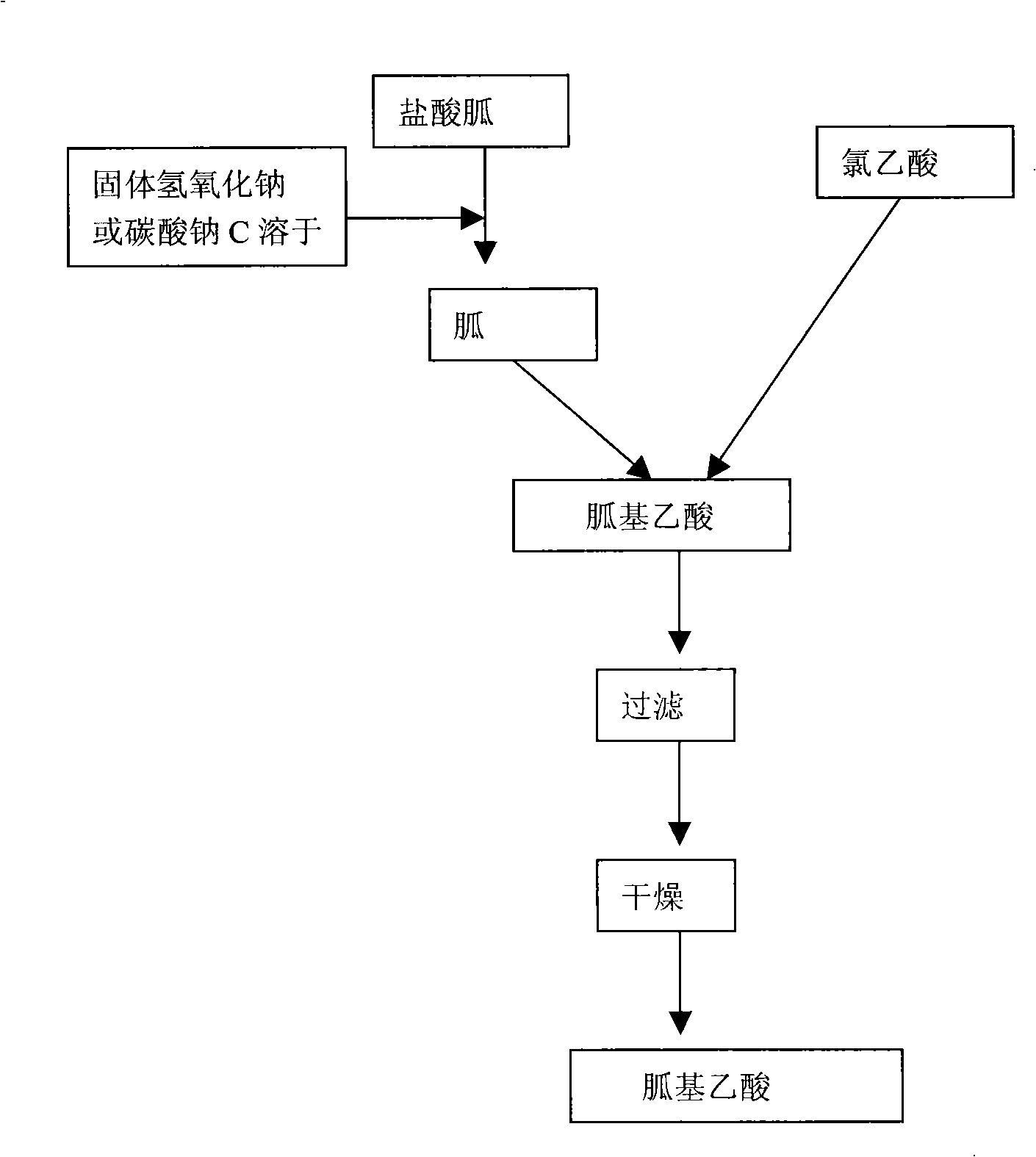
[0067] The synthetic route of guanidinoacetic acid is as follows: figure 2 As shown, the difference between embodiment 2 and embodiment 1 is that chloroacetic acid directly reacts with free guanidine solution to generate guanidinoacetic acid. The specific steps are as follows:
[0068] 1. Preparation of free guanidine solution
[0069] Add 250gNaOH (or 300gNaOH) to 600ml water 2 CO 3 ) stirring and dissolving, cooling to below 20°C, slowly adding 480g of guanidine hydrochloride, stirring and dissolving until transparent. The free guanidine solution A can be obtained.
[0070] Second, the formation of guanidinoacetic acid
[0071] Add 520 g of chloroacetic acid to 520 ml of water and stir at room temperature until dissolved, add dropwise to the free guanidine solution prepared in step 1, stir and react for about 24 hours, and a white solid is formed. Filter, wash with a small amount of water and ethanol, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com