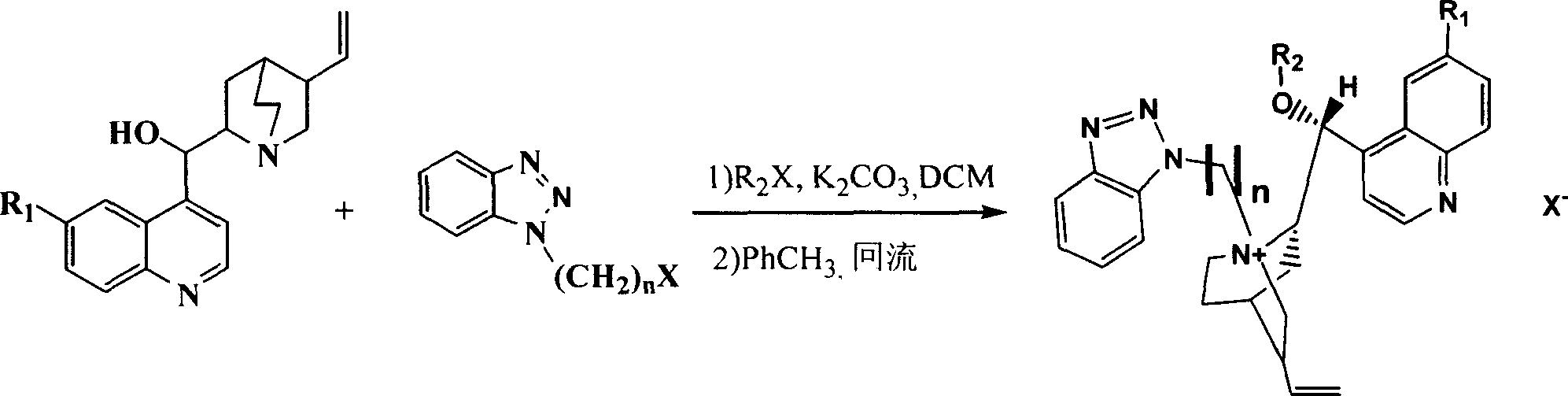Cinchona alkaloid quaternary ammonium salt derivatives as well as preparation method and application thereof
A technology of cinchona alkaloids and quaternary ammonium salts, applied in the direction of cyanide reaction preparation, organic compound preparation, chemical instruments and methods, etc., can solve the problem of narrow substrate range, low catalytic activity of catalyst, and little effect of asymmetric induction Ideal and other problems, to achieve a wide range of substrates, high catalytic efficiency, and good asymmetric induction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
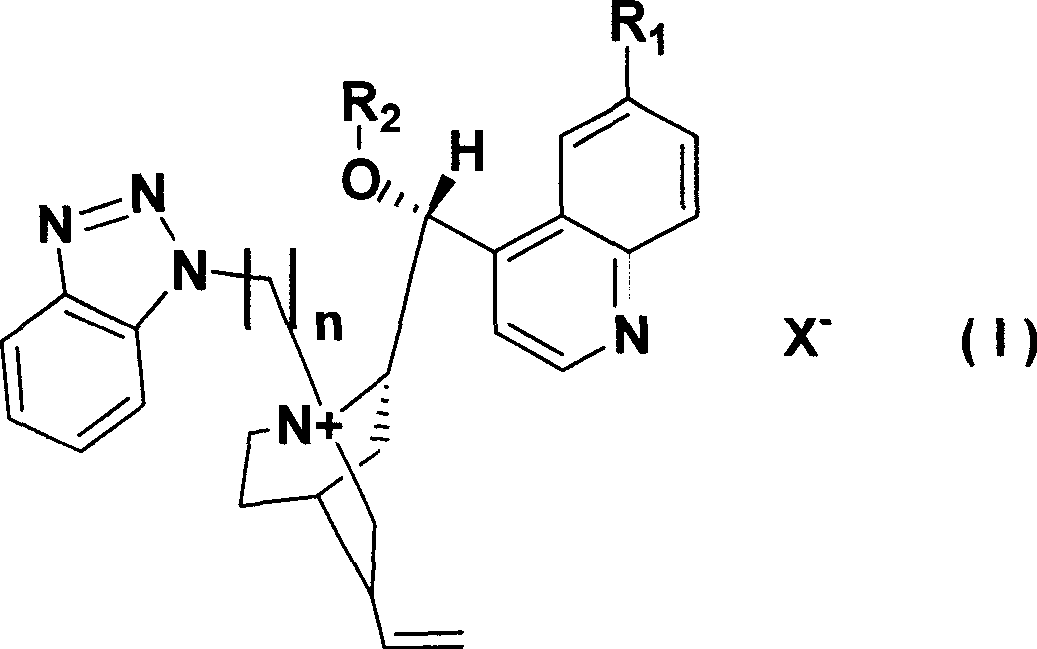
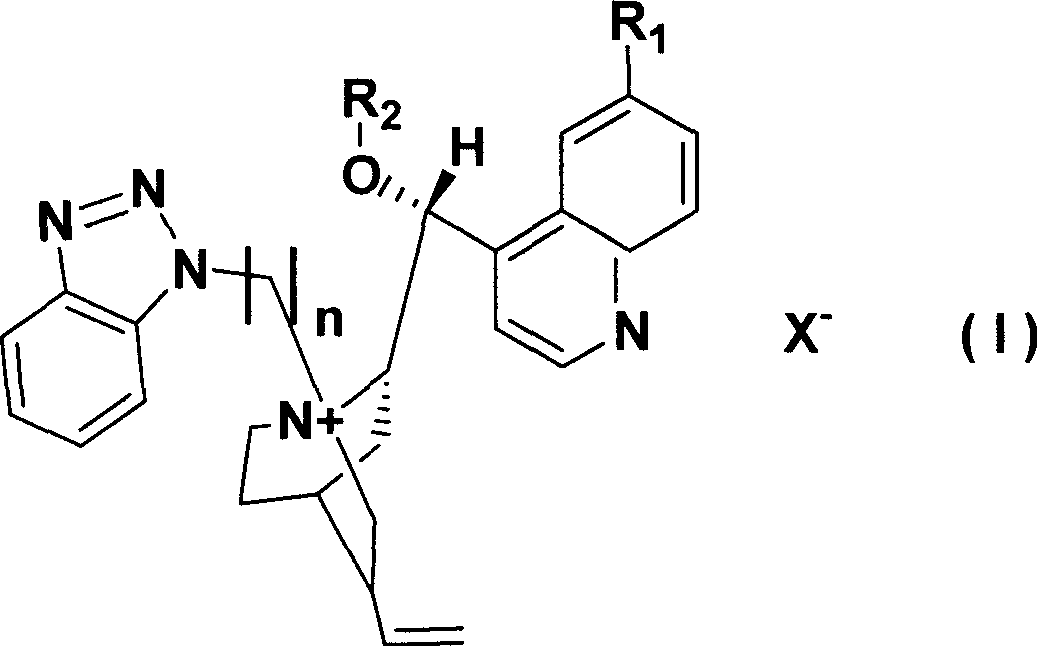
[0027] Embodiment 1 The synthesis of chiral cinchona alkaloid quaternary ammonium salt catalyst L1 (R 1 = H, R 2 =PhCH 2 , X=Cl, n=1)
[0028] Under nitrogen protection, cinchonidine (10mmoL) was added to benzyl bromide (18mmol) and potassium hydroxide (18.8mmol) with a concentration of 50% by mass. Stir vigorously at room temperature for 4h, during which all solids dissolve. Add 30 mL of water for dilution, separate the layers, extract the aqueous layer with dichloromethane, combine the organic phases, dry over anhydrous sodium sulfate, filter, recover the solvent under reduced pressure, and recrystallize the residue with methanol-ether to obtain a light red solid.
[0029] Suspend the above substance in 40mL of toluene, add 1-chloromethylbenzotriazole (10.5mmoL), slowly heat, and reflux for 2h (CH 2 Cl 2 / MeOH as the eluent, TLC to detect the end point of the reaction), cooled to room temperature, precipitated solid, placed overnight in the refrigerator, filtered, the o...
Embodiment 2
[0030] Embodiment 2 The synthesis of chiral cinchona alkaloid quaternary ammonium salt catalyst L2 (R 1 =OCH 3 , R 2 =CH 2 =CHCH 2 , X=Cl, n=1)
[0031] Under nitrogen, quinine (10 mmol) was added to allyl bromide (18 mmol) and 50% potassium hydroxide (18.8 mmol). Stir vigorously at room temperature for 4h, during which all solids dissolve. Add 30 mL of water for dilution, separate the layers, extract the aqueous layer with dichloromethane, combine the organic phases, dry over anhydrous sodium sulfate, filter, recover the solvent under reduced pressure, and recrystallize the residue with methanol-ether to obtain a light red solid.
[0032] Suspend the above substance in 40mL of toluene, add 1-chloromethylbenzotriazole (10.5mmoL), slowly heat, and reflux for 2h (CH 2 Cl 2 / MeOH as the eluent, TLC to detect the end point of the reaction), cooled to room temperature, precipitated solid, placed overnight in the refrigerator, filtered, the obtained crude product was washed w...
Embodiment 3
[0033] Embodiment 3 The synthesis of chiral cinchona alkaloid quaternary ammonium salt catalyst L3 (R 1 =OH, R 2 =C 6 h 9 CH 2 , X=Br, n=2)
[0034] Under nitrogen, hydroxyquinidine (10 mmol) was added to chloromethylcyclohexene (18 mmol) and 50% potassium hydroxide (18.8 mmol). Stir vigorously at room temperature for 4h, during which all solids dissolve. Add 30 mL of water for dilution, separate the layers, extract the aqueous layer with dichloromethane, combine the organic phases, dry over anhydrous sodium sulfate, filter, recover the solvent under reduced pressure, and recrystallize the residue with methanol-ether to obtain a light red solid.
[0035] Suspend the above substance in 40mL toluene, add chloroethyl benzotriazole (10.5mmoL), slowly heat, and reflux for 2h (CH 2 Cl 2 / MeOH as the eluent, TLC to detect the end point of the reaction), cooled to room temperature, precipitated solid, placed overnight in the refrigerator, filtered, the obtained crude product wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com