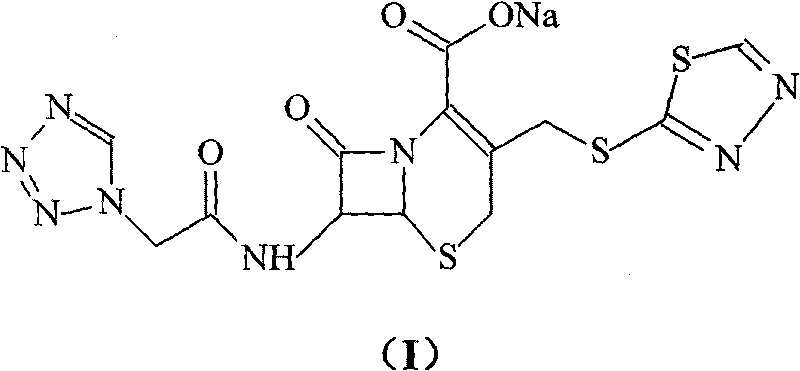
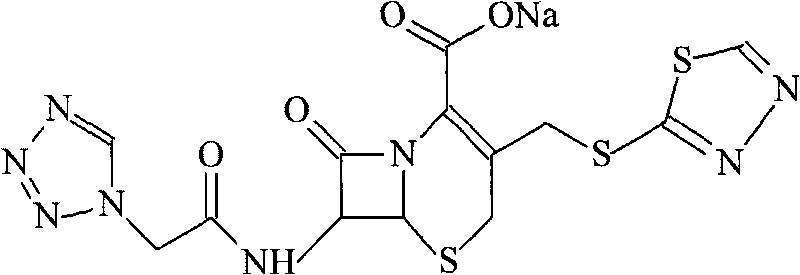
Cefobutazine sodium compound and pharmaceutical composition made therefrom
A technology of ceftezole sodium and refined products, which is applied in the field of purification of ceftezole sodium, can solve the problems of high polymer content of ceftezole sodium, lower content of active ingredients of the drug, improper control of production process, etc., and achieve simple and easy operation OK, long validity period, and solve the effect of poor liquidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Purify ceftezole sodium as follows:
[0034] 1). Dissolve 100g of ceftezole sodium crude product in 1000ml of water, add 2mol / L hydrochloric acid solution, adjust the pH value to 3.0, and stir to precipitate insoluble matter;
[0035] 2). Filter out the insoluble matter, obtain a solid after washing with purified water, then add 1000ml ethanol to the obtained solid to dissolve it, absorb it through D101 macroporous adsorption resin, elute and purify with 500ml chloroform, and collect the eluate ;
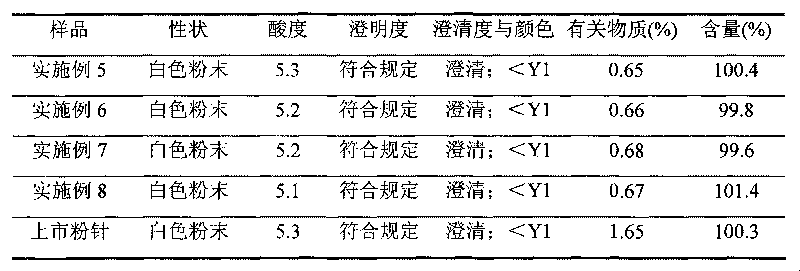
[0036] 3). Regulate the pH value of the eluent with 10% sodium hydroxide solution to be 8.0, separate out solids, wash with 300ml ethanol after centrifugation for 30min, and vacuum-dry at 30°C for 10 hours to obtain 95.5g of ceftezole sodium refined product, The yield was 95.5%, and the purity determined by HPLC was 99.5%.
Embodiment 2
[0038] Dissolve 100g of crude ceftezole sodium in 1000ml of water, add 5mol / L formic acid solution, adjust the pH value to 5.0, stir to precipitate insoluble matter, filter, obtain a solid after washing with purified water, and then add 800ml of dichloromethane to the resulting solid Make it dissolve, absorb by AB-8 macroporous resin, elute and purify with 800ml acetone, collect the eluate; then use 20% sodium acetate solution to adjust the pH value of the eluate to 8.0, precipitate solid, centrifuge for 20min and use Washed with 300ml of ethanol and dried in vacuum at 40°C for 8 hours to obtain 94.7g of refined product of ceftezole sodium with a yield of 94.7%, and a purity of 99.7% as determined by HPLC.
Embodiment 3
[0040]Dissolve 100g of crude ceftezole sodium in 1000ml of water, add 5% sodium dihydrogen phosphate solution, adjust the pH value to 4.5, stir to precipitate insoluble matter, filter, wash with purified water to obtain a solid, and then add 1000ml of methanol to the obtained solid Make it dissolve, absorb with AB-8 macroporous resin, elute and purify with 500ml petroleum ether, collect the eluate; then use 10% ammonia solution to adjust the pH value of the eluate to 9.0, precipitate solid, centrifuge for 10min and use Washed with 300ml of ethanol, dried under vacuum at 35°C for 10 hours to obtain 96.3g of refined ceftezole sodium, with a yield of 96.3%, and a purity of 99.6% as determined by HPLC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com