Pure organic dye containing conjugated units of different heterocyclic rings and derivatives thereof and application thereof in dye-sensitized solar cell
A technology of organic dyes and derivatives, applied in the field of pure organic dyes, can solve the problems of high price, limited practical application of precious metal resources, weak absorption spectrum, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Embodiment 1: the preparation of the pure organic dye that contains different kinds of heterocycles and derivatives conjugated units thereof of chemical structural formula I
[0100] The synthetic route is as follows:
[0101]
[0102] Structural formula is the synthesis of the material of (1):
[0103] In the reactor, 11.14 g (67 mmol) of dithiophene was dissolved in a mixed solvent of 90 ml of chloroform and 40 ml of glacial acetic acid, and cooled to -15°C. Add 13.73 milliliters (268 mmol) of liquid bromine dropwise, rise to room temperature and react for 5 hours, then heat up to reflux for 24 hours, cool to room temperature, add 100 milliliters of 10% potassium hydroxide aqueous solution by mass, extract with chloroform, and use anhydrous magnesium sulfate to dry. After spinning off the solvent, the product (1) was obtained by column chromatography with a yield of 69%.
[0104] Structural formula is the synthesis of the material of (2):
[0105] In the reacto...
Embodiment 2
[0129] Example 2: Preparation of dye-sensitized solar cells from pure organic dyes of chemical structural formula I containing different heterocycles and their derivatives conjugated units
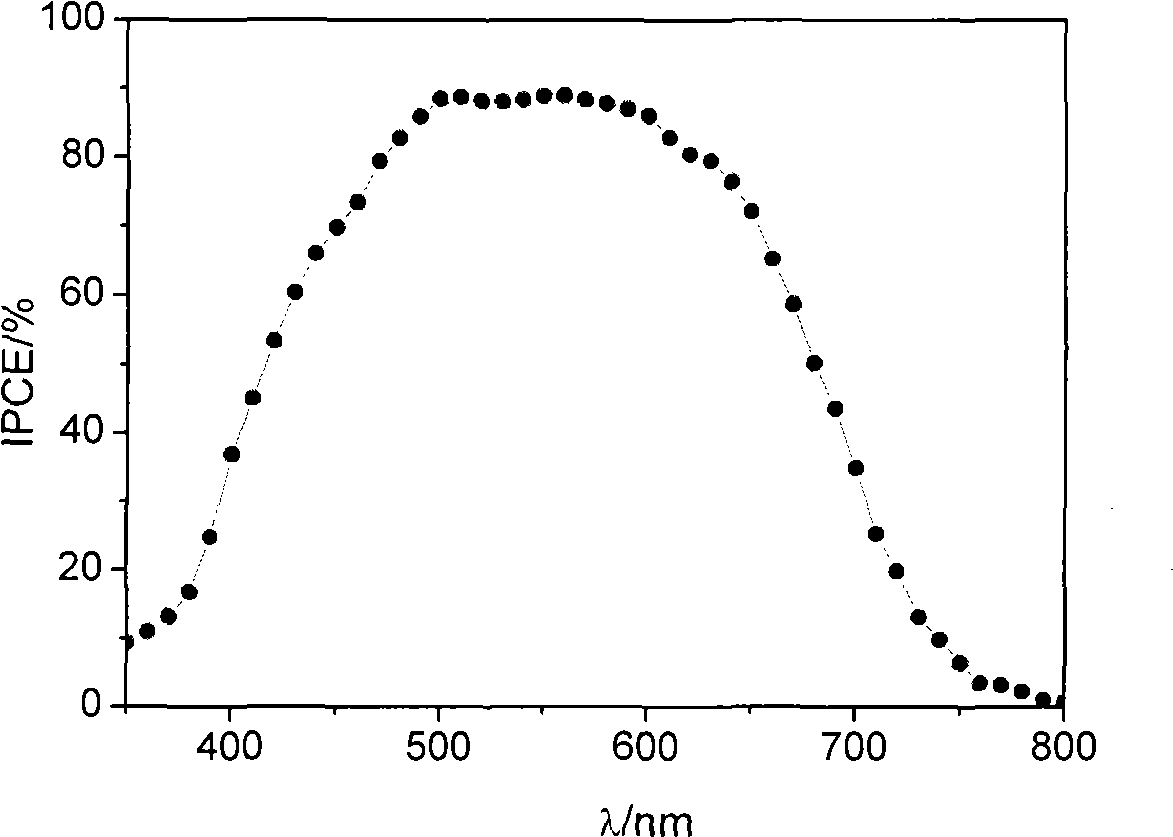
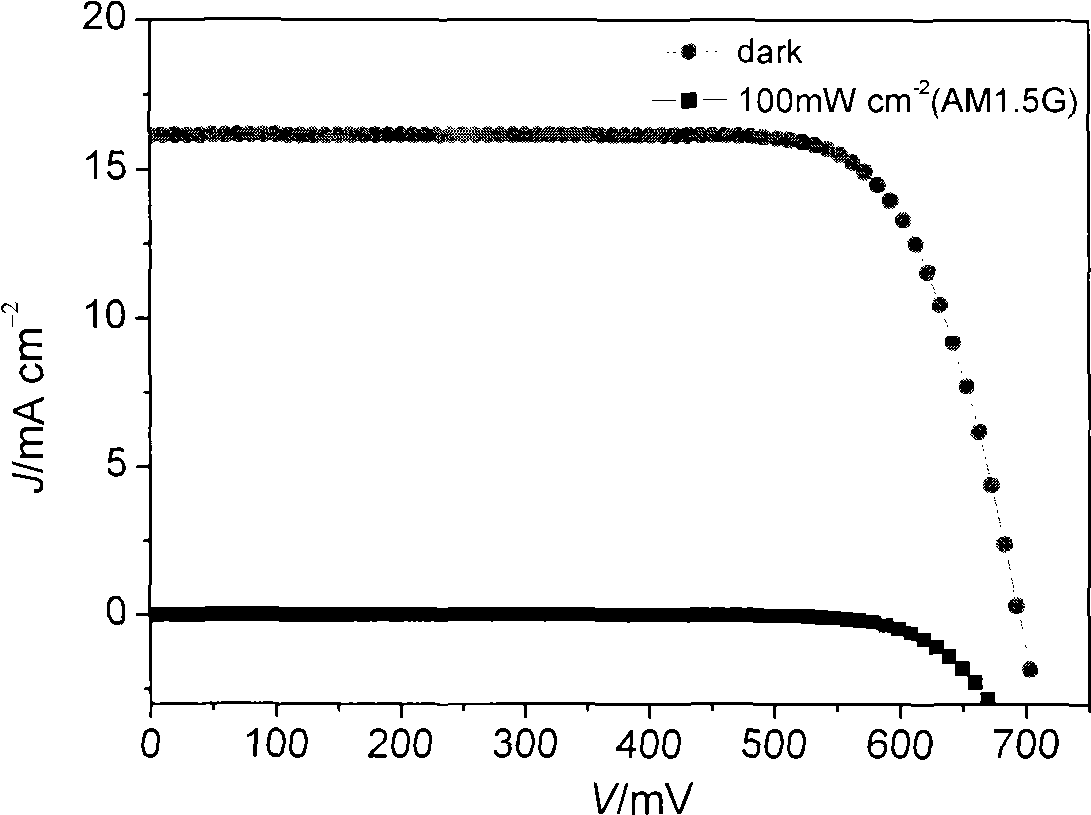
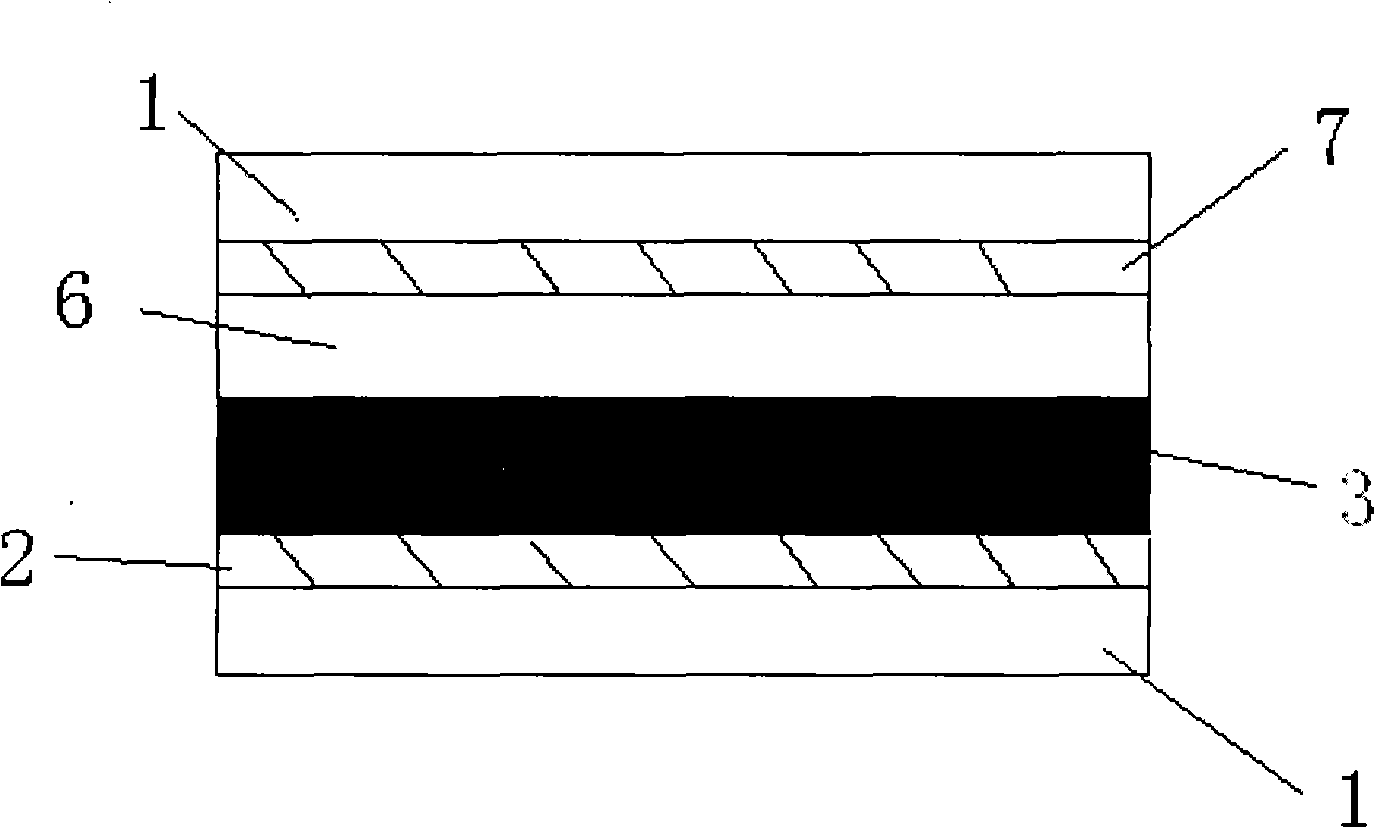
[0130] The photoanode (light-absorbing layer) of the dye-sensitized solar cell adopts a mesoporous double layer, and the underlying film is made of 20nm TiO 2 Composed of nanocrystals, the thickness is 7μm, and the upper film thickness is made of 400nm TiO 2 Composed of light-scattering particles with a thickness of 5 μm. Preparation of TiO 2 Nanocrystalline and TiO 2 For the method of nanostructured double-layer film electrodes, see the article (Wang P. et al., Enhance the Performance of Dye-Sensitized Solar Cells by Co-grafting AmphiphilicSensitizer and Hexadecylmalonic Acid on TiO 2 Nanocrystals, J. Phys. Chem. B., 107, 2003, 14336).
[0131] Prepared TiO 2 The nanostructured double-layer membrane electrode is soaked in acetonitrile / tert-butanol containing 300 μM of the dye of stru...
Embodiment 3
[0133] Example 3: Dye-sensitized solar cells prepared from pure organic dyes of chemical structure II containing different heterocycles and their derivatives conjugated units
[0134]
[0135] The pure organic dyes containing different kinds of heterocyclic rings and their derivatives conjugated units of chemical structural formula II used are 4,4'-dimethylsilyl-2,2'-bithiophene, using the steps of Example 1 and conditional synthesis.
[0136] The dye-sensitized solar cell was prepared according to the method in Example 2, and the parameters of the obtained dye-sensitized solar cell are shown in the appended table of the instruction manual.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Short circuit photocurrent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com