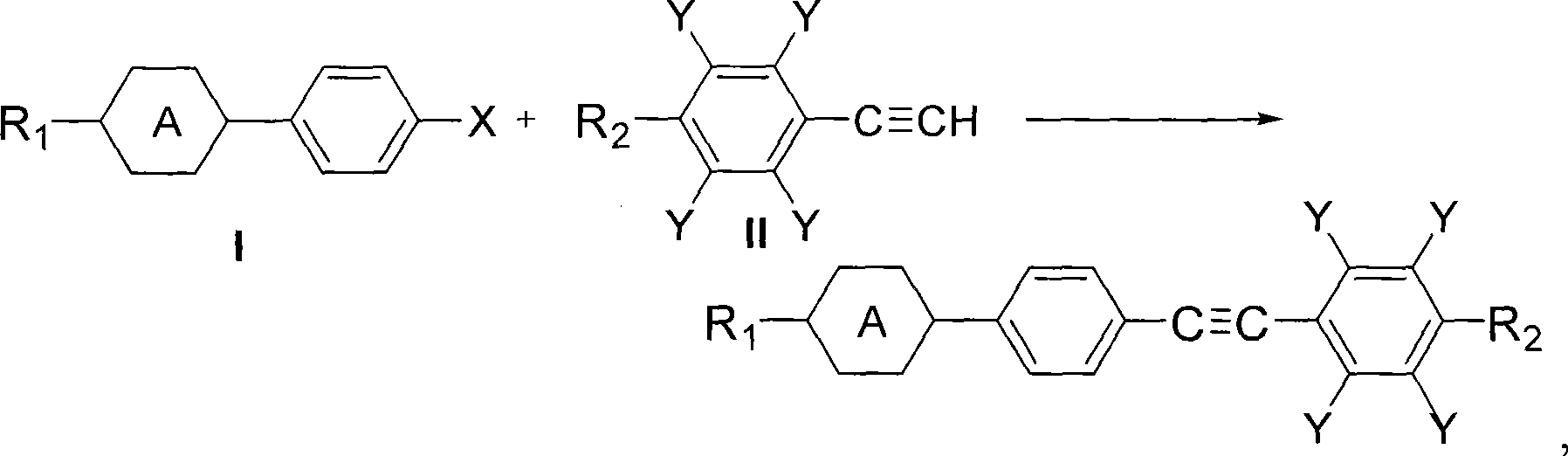
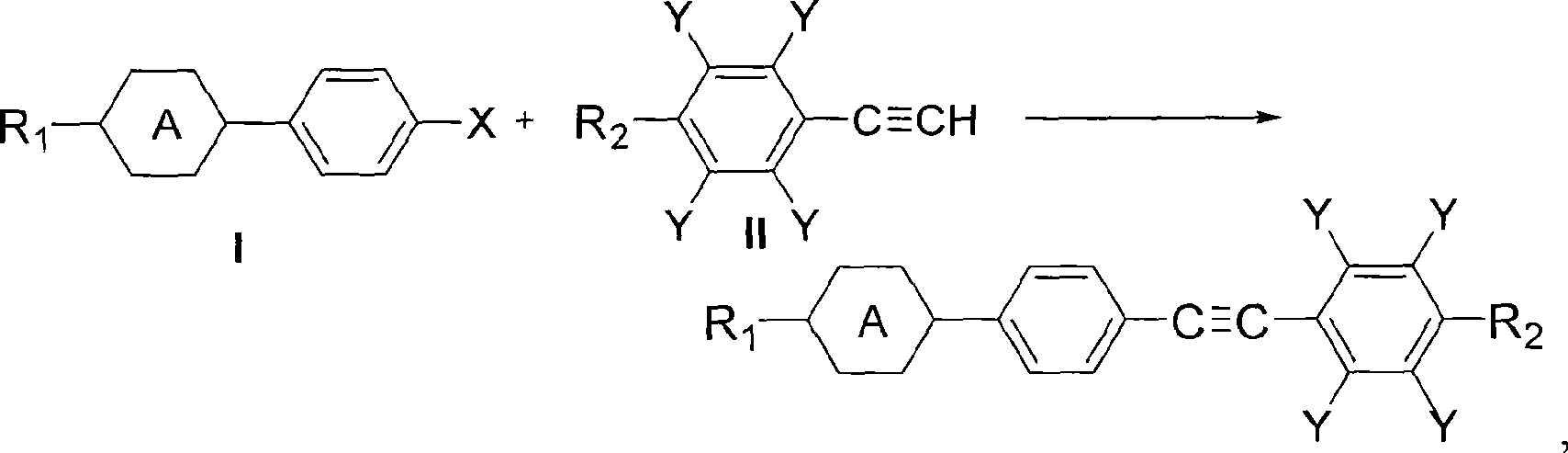
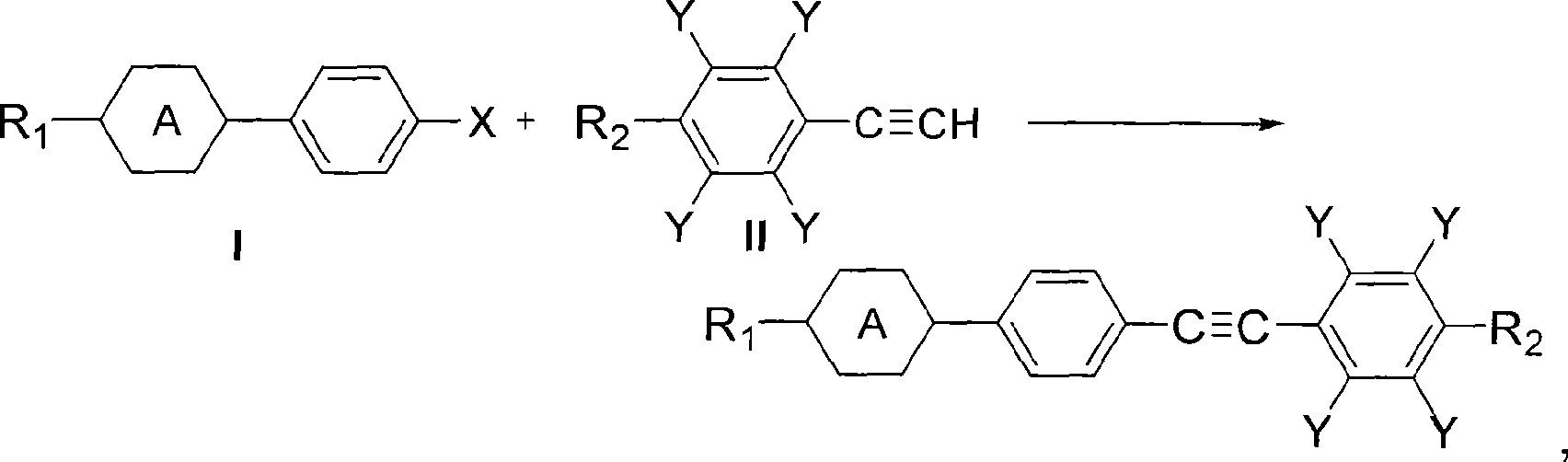
Method for synthesizing diaryl acetylene monomer liquid crystal
A diaryl acetylene and monomer technology, which is applied in the catalytic field of liquid crystal synthesis of diaryl acetylene monomers, can solve the problems of difficult recycling of precious metal catalysts, complicated product purification process, and difficult purification of monomer liquid crystals, etc., and achieves The effect of increasing economic and social benefits, improving synthesis technology, and good catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Embodiment 1——The reaction of propyl cyclohexyl iodobenzene in the mixed solvent of DMF and water
[0056] Add 8.2g (0.025mol) of 4-(4-propylcyclohexyl) iodobenzene, 3.85g (0.0275mol) of 4-methoxyphenylacetylene, 0.05g (0.0003 equivalents) of 5% Pd / C in a 100mL three-necked flask ), CuI 0.1g (0.02 equivalent), PPh 3 0.04g (0.006 equivalent), Et 3 N 8g (3.0 equivalent), DMF 50mL, water 20mL, nitrogen replacement, under the protection of nitrogen, stirred and heated to reflux for 3h, the reaction was completed. Then take a sample for chromatographic analysis, the separation yield: 85%, and the chromatographic purity is 99.6%.
Embodiment 2
[0057] Embodiment 2——The reaction of propyl cyclohexyl iodobenzene in the mixed solvent of acetone and water
[0058] The difference between this example and Example 1 is that the solvent used is a mixed solvent of acetone and water, wherein 50 mL of acetone and 20 mL of water, and other reaction conditions are the same as in Example 1. During the reaction process of this example, a white solid was continuously precipitated. After the reaction was completed, chromatographic analysis was carried out, the separation yield: 90%, and the chromatographic purity: 99.8%.
Embodiment 3
[0059] Embodiment 3 - the reaction of pentyl cyclohexyl iodobenzene in the mixed solvent of acetone and water
[0060] Add 8.9g (0.025mol) of 4-(4-pentylcyclohexyl) iodobenzene, 3.85g (0.0275mol) of 4-methoxyphenylacetylene, 0.05g (0.0003 equivalents) of 5% Pd / C into a 100mL three-necked flask ), CuI 0.1g (0.02 equivalent), PPh 3 0.04g (0.006 equivalent), Et 3 N 8g (3.0 equivalent), acetone 50mL, water 20mL, nitrogen replacement, protection, stirring and heating to reflux for 3h. During the reaction process, a white solid was continuously precipitated, and it was dissolved and sampled for chromatographic analysis. The separation yield was 88%, and the chromatographic purity was 99.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com