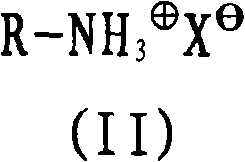Method for the selective catalytic reduction of nitrogen oxides in exhaust gases of vehicles
A nitrogen oxide, selective technology used in chemical instruments and methods, separation methods, exhaust devices, etc., to achieve the effect of reducing the risk of corrosion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
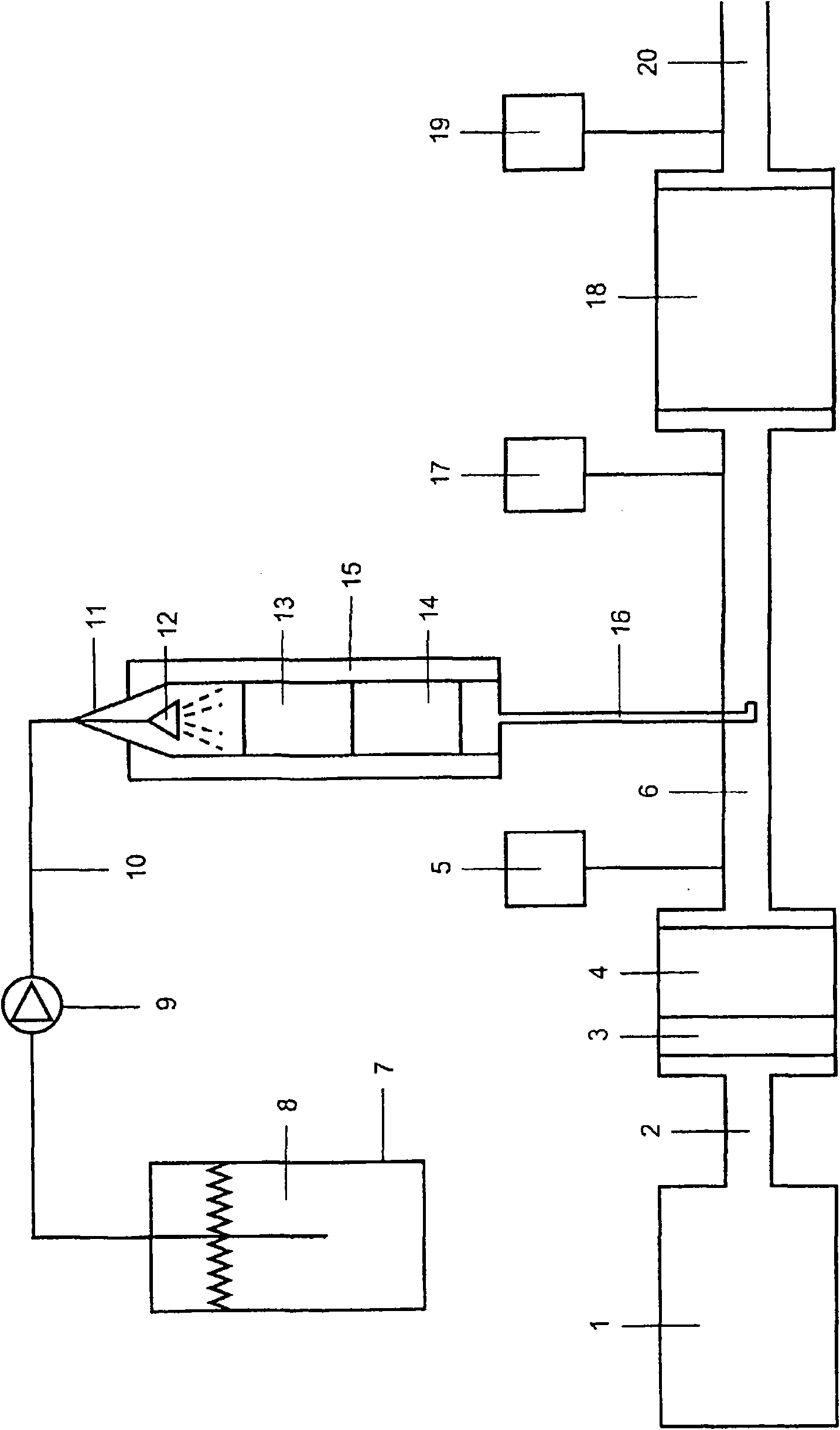
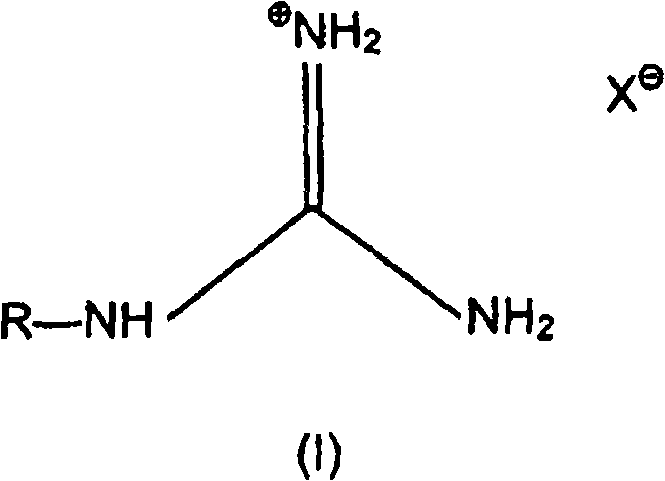
[0042] corresponding to the basis figure 1 Description of the use of 40% by weight guanidine formate in automatic ammonia generators Aqueous solution, (GF) (Fp
[0043] Car engine 1 produces 200Nm 3 / h exhaust gas flow, which is led through the intermediate pipe 2 to the exhaust gas intermediate pipe 6 via the platinum oxidation catalyst 3 and the particle filter 4 . The composition of exhaust gas measured with FTIR-gas analyzer 5 in intermediate pipe 6: nitrogen oxide (NO) of 150ppm; nitrogen dioxide (NO2) of 150ppm 2 ); 7% of carbon dioxide (CO 2 ); 8% of water vapor, 10ppm of carbon monoxide (CO).
[0044] In the storage tank 7 there is the GF-solution 8 , which is sprayed into the reactor 11 by means of the metering pump 9 via the delivery pipe 10 and the nozzle 12 . The reactor 11 consists of a vertically arranged tube of an inner diameter of 51 mm made of austenitic stainless steel heated to 250° C. and provided with a heating jacket 15 . In reactor 11 there ar...
Embodiment 2
[0049] Similar to embodiment 1 operation, but titanium dioxide catalyst 14 is replaced by palladium oxide-titania catalyst, wherein titanium dioxide is replaced with Pd(NO 3 ) 2 The aqueous solution impregnated such that after drying and calcination (5 hours at 500° C.) a catalyst containing 1% by weight of PdO (= about 0.9% by weight of Pd) was produced and which resulted in partial oxidation of carbon monoxide. On the FTIR gas analyzer 17 no increase in the CO concentration can be detected.
Embodiment 3
[0051] The procedure was carried out analogously to Example 1, but instead of the 40% by weight guanidine formate solution a 15% by weight guanidine dicarbonate solution was used. Reactor 11 was also heated at 250° C., catalysts 13 and 14 were the same as those of Example 1.
[0052] No by-products (2 Elevation as expected; NO and NO detectable in gas analyzer 19 2 The concentration was reduced by about 92% to 25 ppm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com