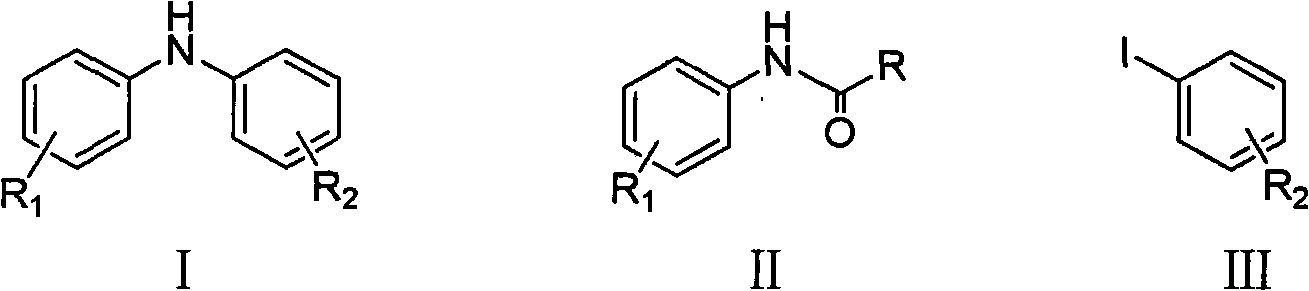
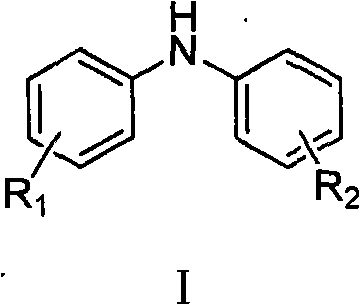
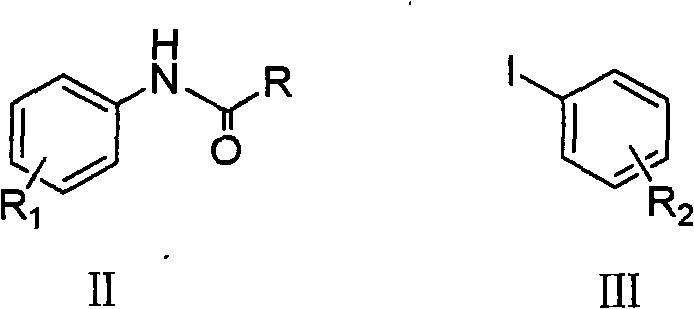
Method for synthesizing copper-catalyzed substituted diphenylamine
A copper compound and compound technology are applied in the field of preparation of organic synthesis intermediates substituted diphenylamine, can solve the problems of difficult industrialization, high price, and difficulty in obtaining products, and achieve the effects of low cost, short reaction time, and low solvent toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1 4, the synthesis of 4'-dimethyldianiline
[0024] In a 100ml reaction flask, add 2.98g (20.0mmol) of N-(p-methylphenyl)acetamide, 30ml of toluene, and 5.23g (24.0mmol) of p-iodotoluene in sequence, stir and dissolve and add 4.49g (40.0mmol) Potassium tert-butoxide, add 198mg (2.0mmol) cuprous chloride (CuCl) and 792mg (4.0mmol) 1,10-phenanthroline (1,10-phenanthroline) again, heat to 130 ℃ and stir reaction for 10 hours, drop to room temperature, wash the reaction solution with water (30ml×3) to neutrality, and recover the toluene, dissolve the residue in 20ml concentrated hydrochloric acid, reflux for 2 hours, neutralize the reaction solution with NaOH, and then use ethyl acetate (15mL×3 ) extraction, and dried over anhydrous sodium sulfate. The solvent is recovered to obtain the crude product. Ethanol / water recrystallization gave 3.25 g of the product 4,4'-dimethyldiphenylamine, yield: 82.5%. mp: 79-80°C.
Embodiment 2
[0025] The synthesis of embodiment 2 4-methyl-4'-methoxydiphenylamine
[0026] In a 100ml reaction flask, sequentially add 2.98g (20.0mmol) of N-(p-methylphenyl)acetamide, 30ml of xylene, and 5.62g (24.0mmol) of p-methoxyiodobenzene, stir and dissolve, then add 10.65g (40.0mmol) Potassium phosphate trihydrate, then add 381mg (2.0mmol) cuprous iodide (CuI) and 456.8mg (4.0mmol) cyclohexanediamine, heat to 160°C and stir under reflux for 10 hours, then cool to room temperature, water (30ml×3) wash the reaction solution to neutrality, after recovery of xylene, dissolve the residue in 20ml concentrated hydrochloric acid, and after reflux reaction for 2 hours, neutralize the reaction solution with NaOH, then extract with ethyl acetate (15mL×3) , and recover the solvent after drying with anhydrous sodium sulfate to obtain the crude product. Ethanol / water recrystallization gave 3.60 g of the product 4-methyl-4'-methoxydiphenylamine, yield: 84.4%. mp: 82-84°C.
Embodiment 3
[0027] The synthesis of embodiment 3 4-methyldiphenylamine
[0028] In a 100ml reaction flask, add 2.98g (20.0mmol) of N-(p-methylphenyl)acetamide, 30ml of xylene, and 4.90g (24.0mmol) of iodobenzene in sequence, stir and dissolve and add 4.49g (40.0mmol) Potassium tert-butoxide, then add 381mg (2.0mmol) cuprous iodide (CuI) and 961.2mg (4.0mmol) N, N'-dibenzylethylenediamine, heat to 160 ° C and stir under reflux for 10 hours, down to At room temperature, wash the reaction solution with water (30ml×3) to neutrality, recover xylene, dissolve the residue in 20ml concentrated hydrochloric acid, and after reflux reaction for 2 hours, neutralize the reaction solution with NaOH, and then use ethyl acetate (15mL×3 ) extraction, and after drying with anhydrous sodium sulfate, the solvent was recovered to obtain a crude product. After recrystallization, 2.75 g of the product 4-methyldianiline was obtained, yield: 75.1%. mp: 88-89°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com