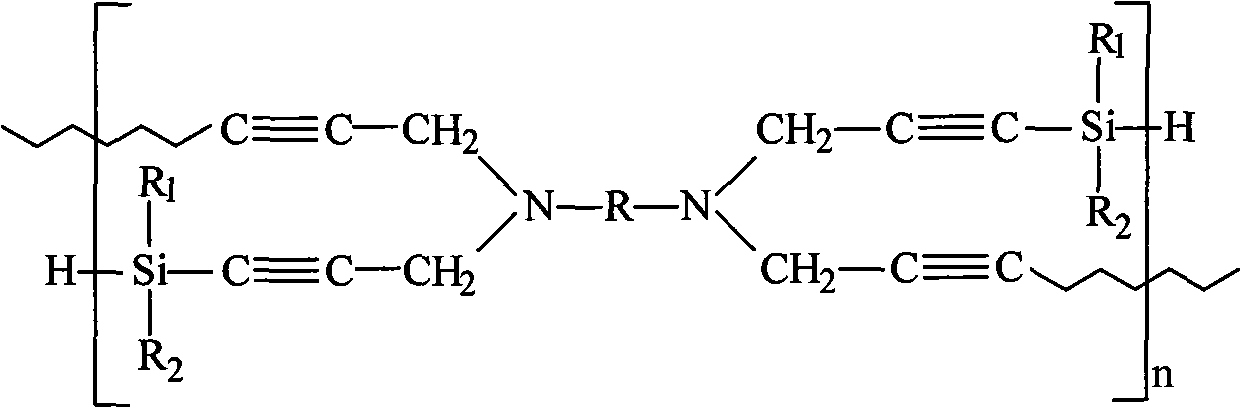
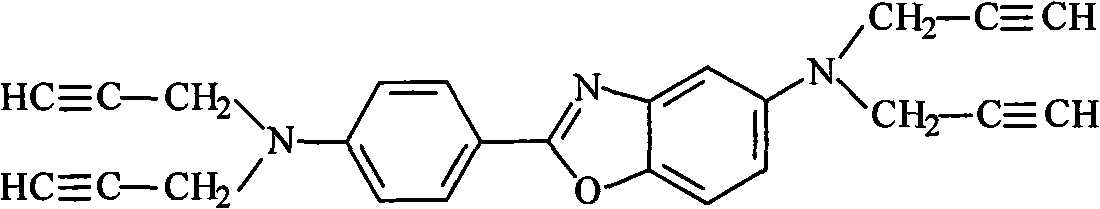
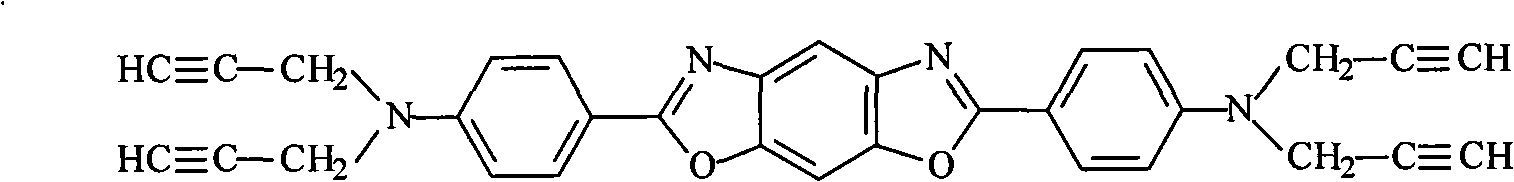
High-temperature resistant polymer with main chain containing phenylsilylene-propargyl-benzoxazole ring and preparation method thereof
A technology of benzoxazole ring and silyl methylene, which is applied in the main chain containing silyl methylene-propargyl-benzoxazole ring polymer and its preparation field, which can solve the problem of harsh synthesis reaction conditions and obstacles to development Application, high cost of raw materials, etc., to achieve the effect of low price, increased cross-linking density, and easy access
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1: Poly(phenylsilane-4,4'-N,N,N',N'-tetrapropargyl-5-amino-2-(p-aminophenyl)benzoxazole)
[0045] 0.377g (1mmol) N, N, N', N'-tetrapropargyl-5-amino-2-(p-aminobenzene) benzoxazole (propargyl compound of aromatic benzoxazole ring) Add the solution of 2.3ml diethylene glycol dimethyl ether (solvent) into the reaction vessel, gradually add 11.4mg (0.3mmol) lithium aluminum hydride LiAlH under stirring 4 (catalyst) and 0.648g (6mmol) of phenylsilane (silicon hydrogen compound), after being thoroughly mixed, under the protection of an inert gas, the temperature was raised to 120°C, and the reaction was carried out at normal pressure for 24h.
[0046] After the reaction finishes, 2.3ml of toluene and 2.3ml of 1mol / L hydrochloric acid are sequentially added to the reaction vessel at room temperature, fully stirred, liquid-separated, the lower aqueous phase is removed, and 6ml of deionized water is added to the upper organic phase. Separate the liquid, remove the lowe...
Embodiment 2
[0052] Example 2: Poly(phenylsilane-4,4'-N,N,N',N'-tetrapropargyl-1,4-phenylene-di-(5-aminobenzoxazole))
[0053] 1.976g (4mmol) N, N, N', N'-tetrapropargyl-5-amino-2-(p-aminobenzene) benzoxazole (propargyl compound of aromatic benzoxazole ring) Add the solution of 3.77ml diethylene glycol dimethyl ether (solvent) into the reaction vessel, gradually add 19mg (0.5mmol) lithium aluminum hydride LiAlH under stirring 4 (catalyst) and 1.08 g (10 mmol) of phenylsilane (silicon hydrogen compound), after being thoroughly mixed, under the protection of an inert gas, the temperature was raised to 120° C., and the reaction was carried out at normal pressure for 24 hours.
[0054] After the reaction, add 4ml of toluene and 4ml of 1mol / L hydrochloric acid to the reaction vessel in sequence at room temperature, stir fully, separate the liquids, remove the lower aqueous phase, add 15 ml of deionized water to the upper organic phase, and then separate the liquids , remove the lower aqueous...
Embodiment 3
[0057] Example 3: Poly(phenylsilane-4,4'-N,N,N',N'-tetrapropargyl-5-amino-2-(p-aminophenyl)benzoxazole)
[0058] 7.54g (20mmol) N, N, N', N'-tetrapropargyl-5-amino-2-(p-aminobenzene) benzoxazole (propargyl compound of aromatic benzoxazole ring) Add the solution of 30.2ml diethylene glycol dimethyl ether (solvent) into the reaction vessel, gradually add 0.152g (4mmol) lithium aluminum hydride LiAlH under stirring 4 (catalyst) and 8.64g (80mmol) of phenylsilane (silicon hydrogen compound), after being thoroughly mixed, under the protection of an inert gas, the temperature was raised to 120°C, and the reaction was carried out at normal pressure for 30h.
[0059] After the reaction is over, add 30.2ml of toluene and 30.2ml of 1mol / L hydrochloric acid into the reaction vessel in sequence at room temperature, fully stir for 20min, separate the liquids, remove the lower aqueous phase, and add 40ml of deionized water to the upper organic phase , and then separate the liquid, remove...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com