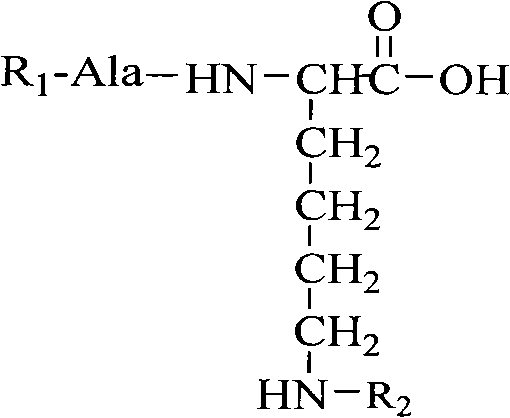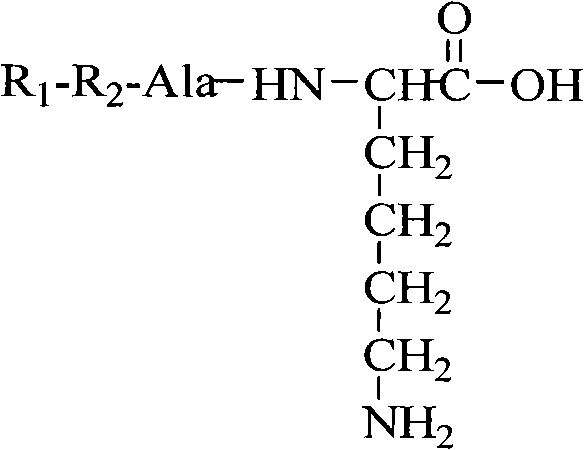Oligopeptide with thrombolysis activity, preparation method and application thereof
An active and thrombolytic technology, applied in the treatment of cerebral thrombosis and in the field of clinical treatment of cerebral thrombosis, oligopeptides, can solve the problems of eNOS and nNOS activity increase and expression upregulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 14
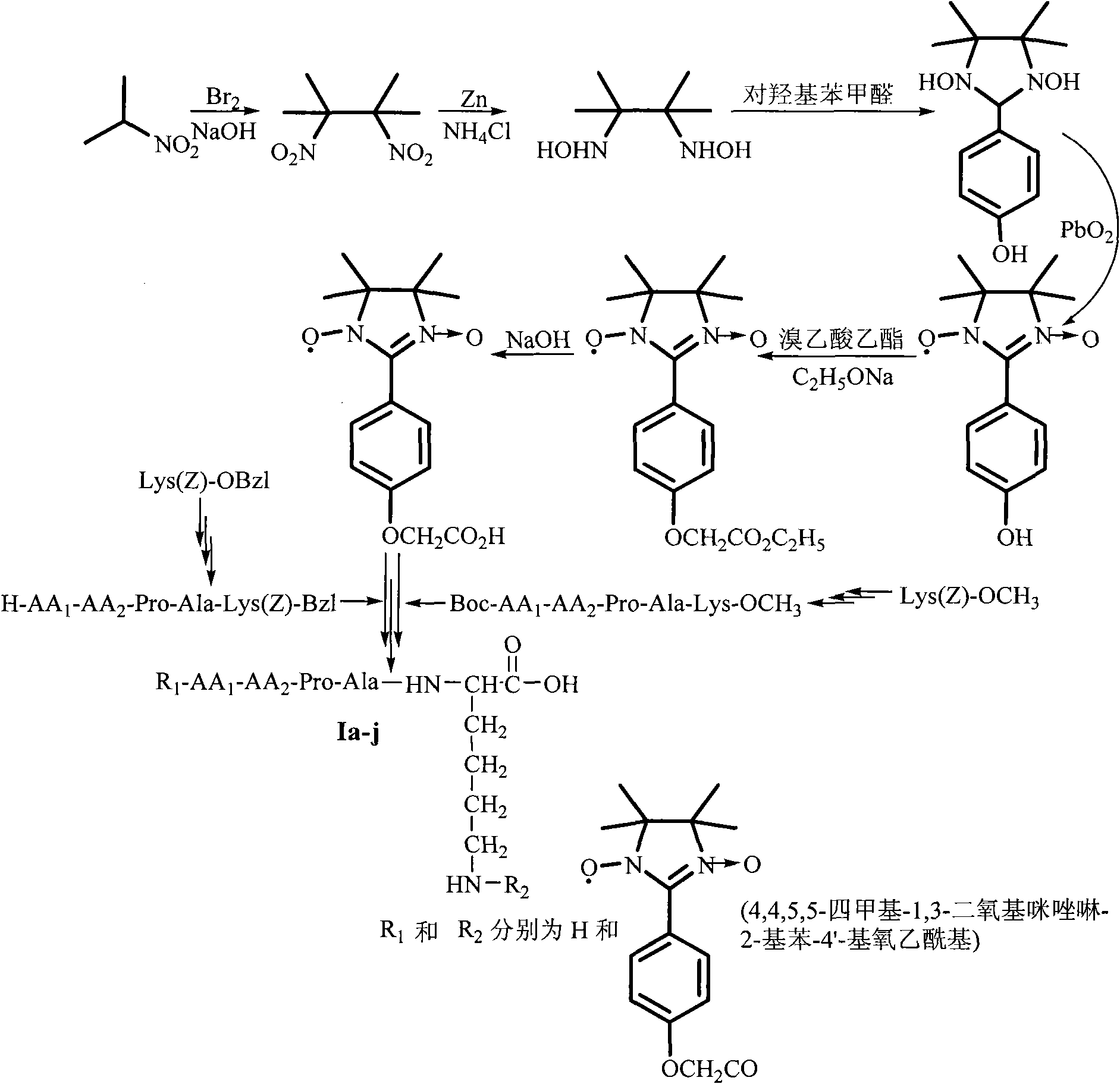
[0032] Embodiment 14,4,5, the preparation of 5-tetramethyl-1,3-dioxyimidazolin-2-ylbenzene-4'-yloxyacetic acid
[0033] 1) Preparation of 2,3-dimethyl-2,3-dinitrobutane
[0034] 69g (0.78mol, 70ml) of 2-nitropropane was added to 130ml of NaOH (6mol / l) aqueous solution. Under stirring conditions in an ice-salt bath, 20ml (0.38mol) of Br was added dropwise 2 , Add dropwise within 1hr. Then 240ml of ethanol was added. The reaction mixture was stirred at reflux at 90°C for 3 hr, and flake insoluble matter appeared. The reaction mixture was poured into 800 ml of ice water while heating. After suction filtration, 55 g (81%) of white flaky crystals were obtained, mp 110-112°C.
[0035] 2) Preparation of 2,3-dimethyl-2,3-dihydroxyaminobutane
[0036] 7.0g (40mmol) 2,3-dimethyl-2,3-dinitrobutane and 4.0g NH 4 Cl was suspended in 80ml ethanol (50%) solution. Stir under ice bath. 16.0 g zinc powder was added within 3 hrs. After the zinc powder was added, the ice bath was remove...
Embodiment 2B
[0045] The preparation of embodiment 2Boc-Pro-Ala-Lys(Z)-OBzl
[0046] 1) Preparation of Boc-Ala-Lys(Z)-OBzl
[0047] Dissolve 473mg (2.5mmol) Boc-Ala-OH in 10ml anhydrous tetrahydrofuran (THF), add 10ml 338mg (2.5mmol) N-hydroxybenzotriazole (HOBt) and 619mg (3.0mmol) dicyclohexyl Carbonyldiimide (DCC) in anhydrous THF. The reaction mixture was stirred in an ice bath for 20 minutes to obtain the corresponding active ester solution for use.
[0048] 936mg (2.3mmol) HCl·Lys(Z)-OBzl and 232mg (2.3mmol) N-methylmorpholine were first miscible with 6ml of anhydrous THF, and then with the active ester solution to be used above. The resulting reaction mixture was reacted at room temperature for 24 hours. TLC (developer chloroform:methanol=20:1) showed that HCl·Lys(Z)-OBzl disappeared. The reaction mixture was concentrated to dryness under reduced pressure, the residue was dissolved in ethyl acetate, and the insoluble matter was filtered off. The filtrate was washed successively ...
Embodiment 3B
[0056] The preparation of embodiment 3Boc-Arg(Tos)-Pro-Ala-Lys(Z)-OBzl
[0057] 1) Preparation of HCl Pro-Ala-Lys(Z)-OBzl
[0058] A mixture of 1.596g (2.5mmol) Boc-Pro-Ala-Lys(Z)-OBzl and 15ml hydrogen chloride-ethyl acetate solution (4N) was stirred at room temperature for 3 hours, TLC (developing agent chloroform:methanol=10:1) showed Boc-Pro-Ala-Lys(Z)-OBzl disappears. Drain the reaction solution with a water pump, add ethyl acetate, and drain the reaction solution with a water pump, repeat 5 times. The residue was soaked and washed with anhydrous ether, and the reaction solution was drained again with a water pump, and this was repeated 5 times. The obtained title compound was directly used in the next reaction.
[0059] 2) Preparation of Boc-Arg(Tos)-Pro-Ala-Lys(Z)-OBzl
[0060] Dissolve 1.073g (2.5mmol) Boc-Arg(Tos)-OH in 10ml of anhydrous THF, and add 10ml of anhydrous THF solution of 338mg (2.5mmol) HOBt and 619mg (3mmol) DCC under ice cooling. The reaction mixtu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The inside diameter of | aaaaa | aaaaa |
| Tube chief | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com