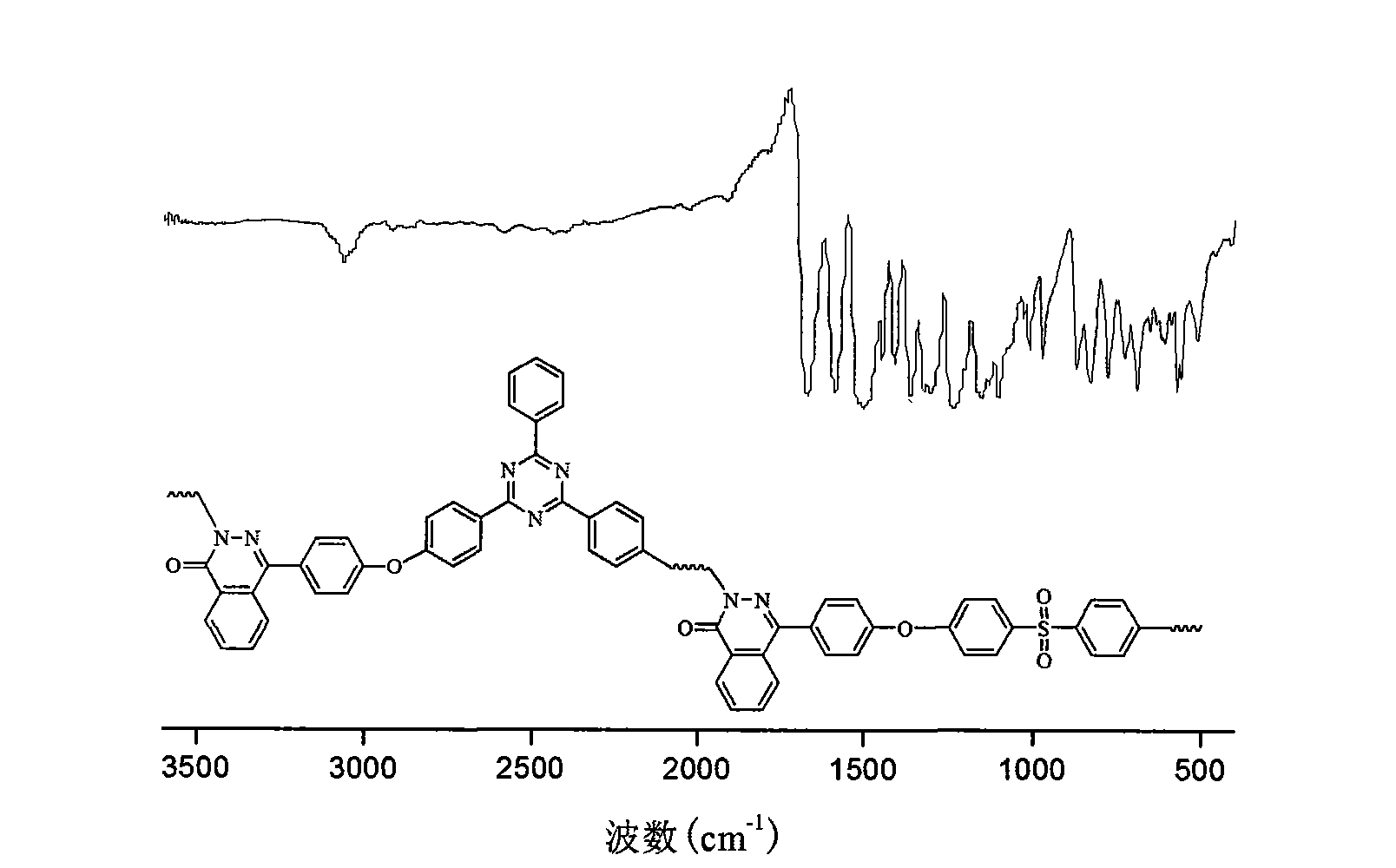
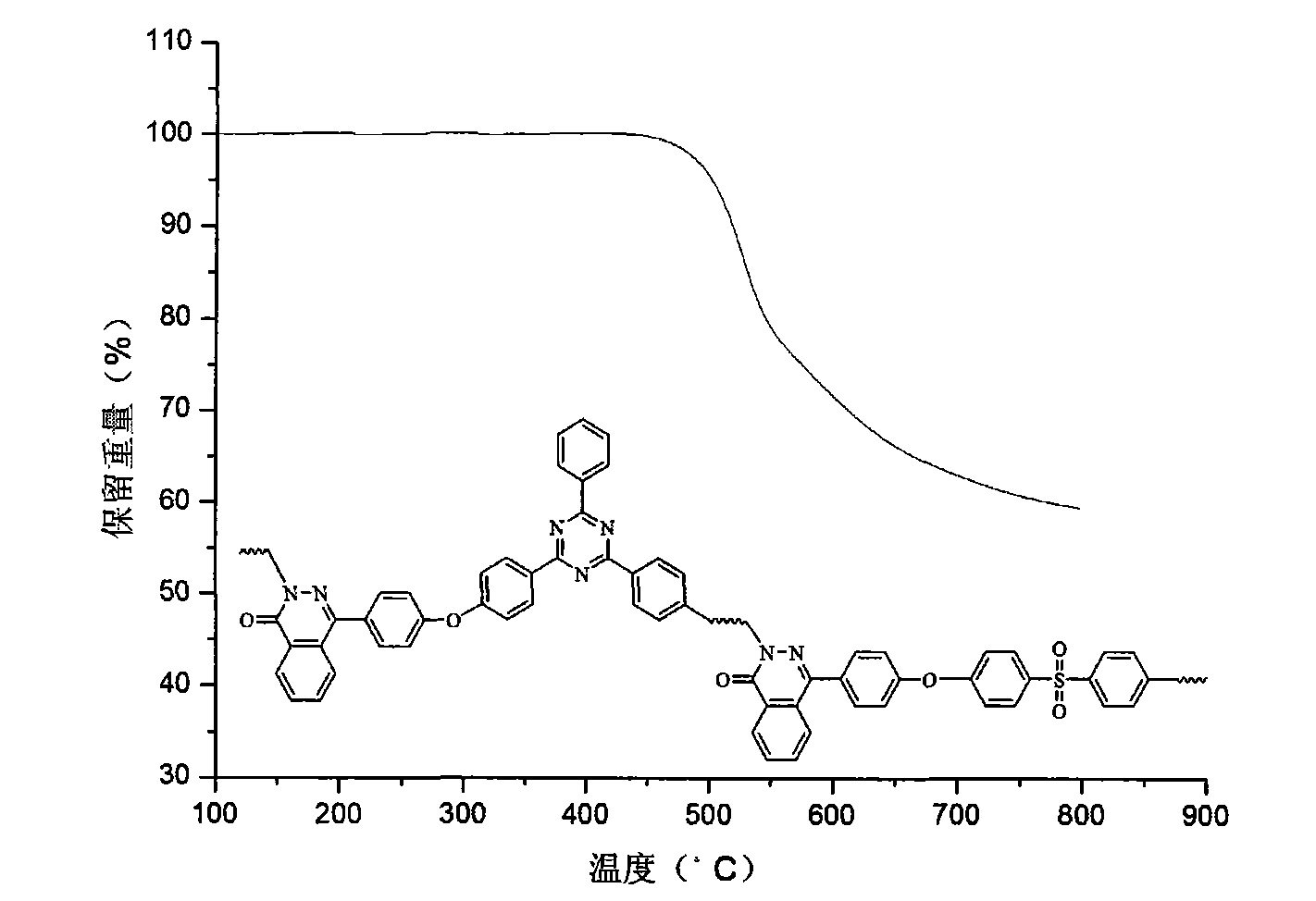
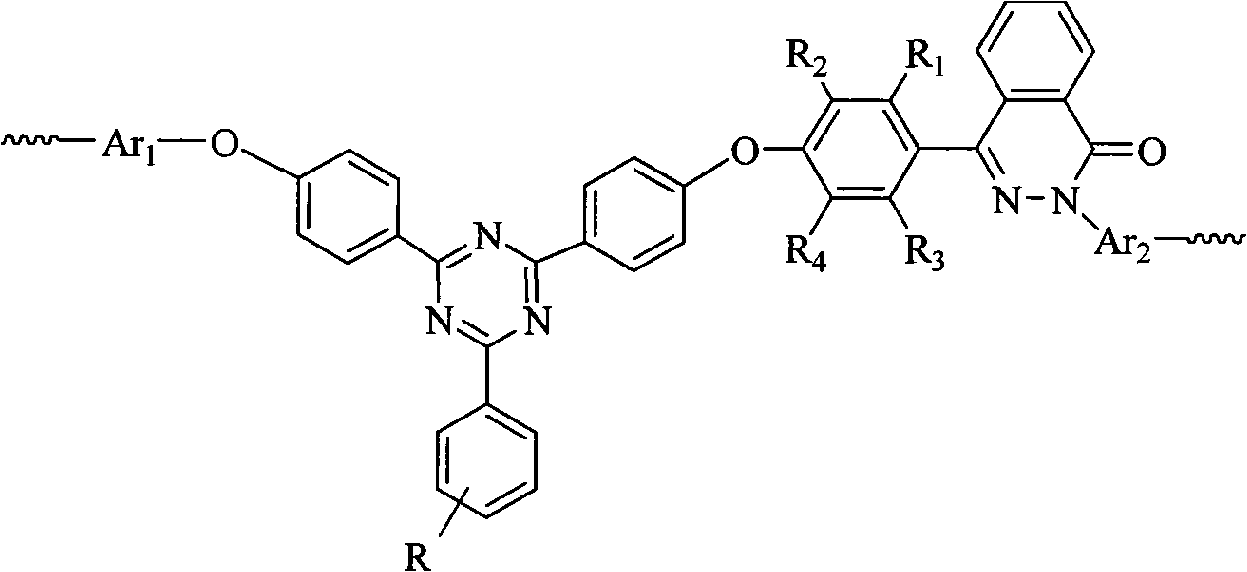
Polyarylether containing triaryl s-triazine ring and phthalazone diphenyl structure and preparation method thereof
A technology of naphthalenone biphenyl and s-triazine, which is applied in the field of polymer science to achieve the effects of soluble film-forming properties, improved solubility, and excellent thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] In a dry 100mL three-neck flask equipped with stirring, reflux condenser, water separator and air duct, add 5mmol of 4-(4-hydroxy-phenyl)-2H-phthalazin-1-one, 6mmol of potassium carbonate, 2-phenyl-4,6-bis(4-fluorophenyl)-1,3,5-s-triazine (BFPT) 2mmol, 4,4'-dichlorodiphenylsulfone 3mmol, toluene 25mL and sulfolane 2mL. Under nitrogen protection, toluene was azeotropically refluxed with water for 3 hours at 140°C, and a large amount of water was evaporated and separated. Then the temperature was raised to 150°C to distill off the toluene, and the temperature was gradually raised to 200°C to continue the reaction. After about 2 hours, the system became viscous, and 0.5 mL of sulfolane was added. After 6-8 hours of high-temperature polymerization, the reaction system turns grayish brown, and the viscosity of the system increases significantly. After the reaction is over, add sulfolane to dilute the reaction system. The polymer solution is stirred at high speed, and boili...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com