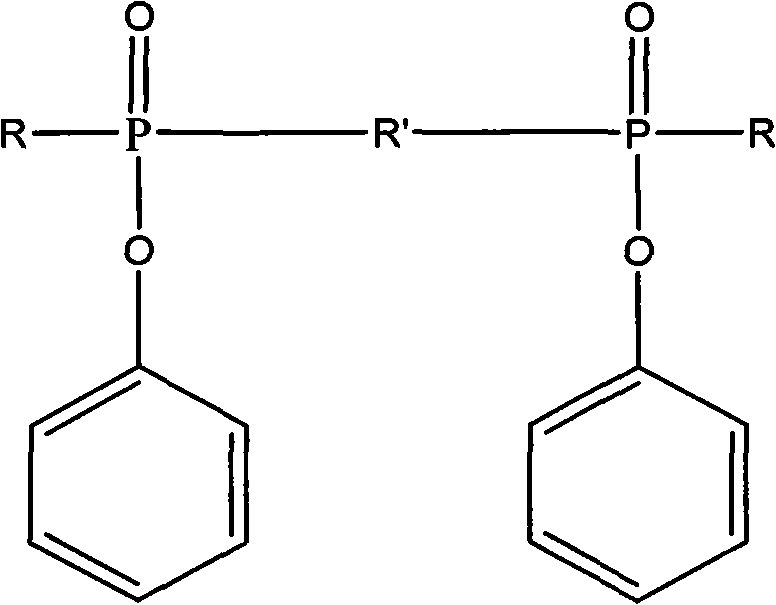
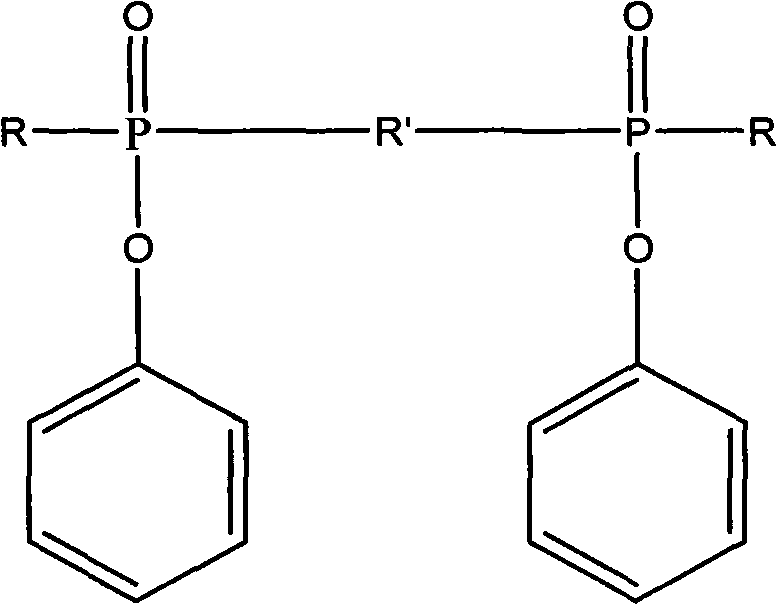
Ultraviolet light-cured phosphorus-nitrogen-containing halogen-free flame retardant and preparation method thereof
A technology of ultraviolet light and flame retardants, applied in the field of flame retardants, can solve the problems of low flame retardant efficiency and complicated preparation steps, and achieve the effects of high production efficiency, good fire protection and good compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] 1. Preparation of UV-curable phosphorus-nitrogen-containing halogen-free flame retardant
[0021] In a 250ml three-neck flask equipped with mechanical stirring and a constant pressure dropping funnel, add 21.1g (0.1mol) phenyl phosphate dichloride and 80ml tetrahydrofuran, stir in an ice bath, and after 10 minutes, add 21.2g (0.21mol) Triethylamine, and a mixed solution of 11.6 g (0.1 mol) of hydroxyethyl acrylate and 20 ml of tetrahydrofuran was slowly added dropwise within 2 hours under continuous stirring, and the reaction was continued for 4 hours. Then 4.307 g (0.05 mol) of piperazine was dissolved in 80 ml of tetrahydrofuran, and slowly dropped into the above reaction system under constant stirring, and reacted for 8 hours. Suction filtration under reduced pressure to remove the triethylamine salt, and distillation under reduced pressure to remove the solvent, and the obtained brown-yellow viscous transparent liquid is the product of the present invention. The yi...
Embodiment 2
[0030] In a 250ml three-necked flask equipped with mechanical stirring and a constant pressure dropping funnel, add 21.1g (0.1mol) phenyl phosphate dichloride and 80ml methylene chloride, stir in an ice bath, and after 10 minutes, add 21.2g (0.21 mol) triethylamine, then slowly dropwise added a mixed solution of 11.6 g (0.1 mol) of hydroxyethyl acrylate and 20 ml of dichloromethane in 2 hours under continuous stirring, and continued to react for 4 hours. Then 4.307 g (0.05 mol) of piperazine was dissolved in 60 ml of dichloromethane, and slowly dropped into the above reaction system under constant stirring, and reacted for 8 hours. Suction filtration under reduced pressure to remove the triethylamine salt, distillation under reduced pressure to remove the solvent, and the viscous transparent liquid obtained is the product of the present invention. The yield was 88%.
[0031] The obtained product was analyzed by nuclear magnetic resonance spectrum. The peak position of the che...
Embodiment 3
[0034] In a 250ml three-neck flask equipped with mechanical stirring and a constant pressure dropping funnel, add 21.1g (0.1mol) phenyl phosphate dichloride and 80ml tetrahydrofuran, stir in an ice bath, and after 10 minutes, add 21.2g (0.21mol) Triethylamine, and a mixed solution of 11.6 g (0.1 mol) of hydroxyethyl acrylate and 20 ml of tetrahydrofuran was slowly added dropwise within 2 hours under continuous stirring, and the reaction was continued for 4 hours. Then, 5.407 g (0.05 mol) of m-phenylenediamine was dissolved in 80 ml of tetrahydrofuran, and slowly dropped into the above reaction system under constant stirring, and reacted for 8 hours. Suction filtration under reduced pressure to remove the pyridinium salt, and distillation under reduced pressure to remove the solvent, and the obtained brown viscous transparent liquid is the product of the present invention. The yield was 85%.
[0035] The obtained product was analyzed by nuclear magnetic resonance spectrum. The...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum heat release rate | aaaaa | aaaaa |
| Initial thermal decomposition temperature | aaaaa | aaaaa |
| Maximum heat release rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com