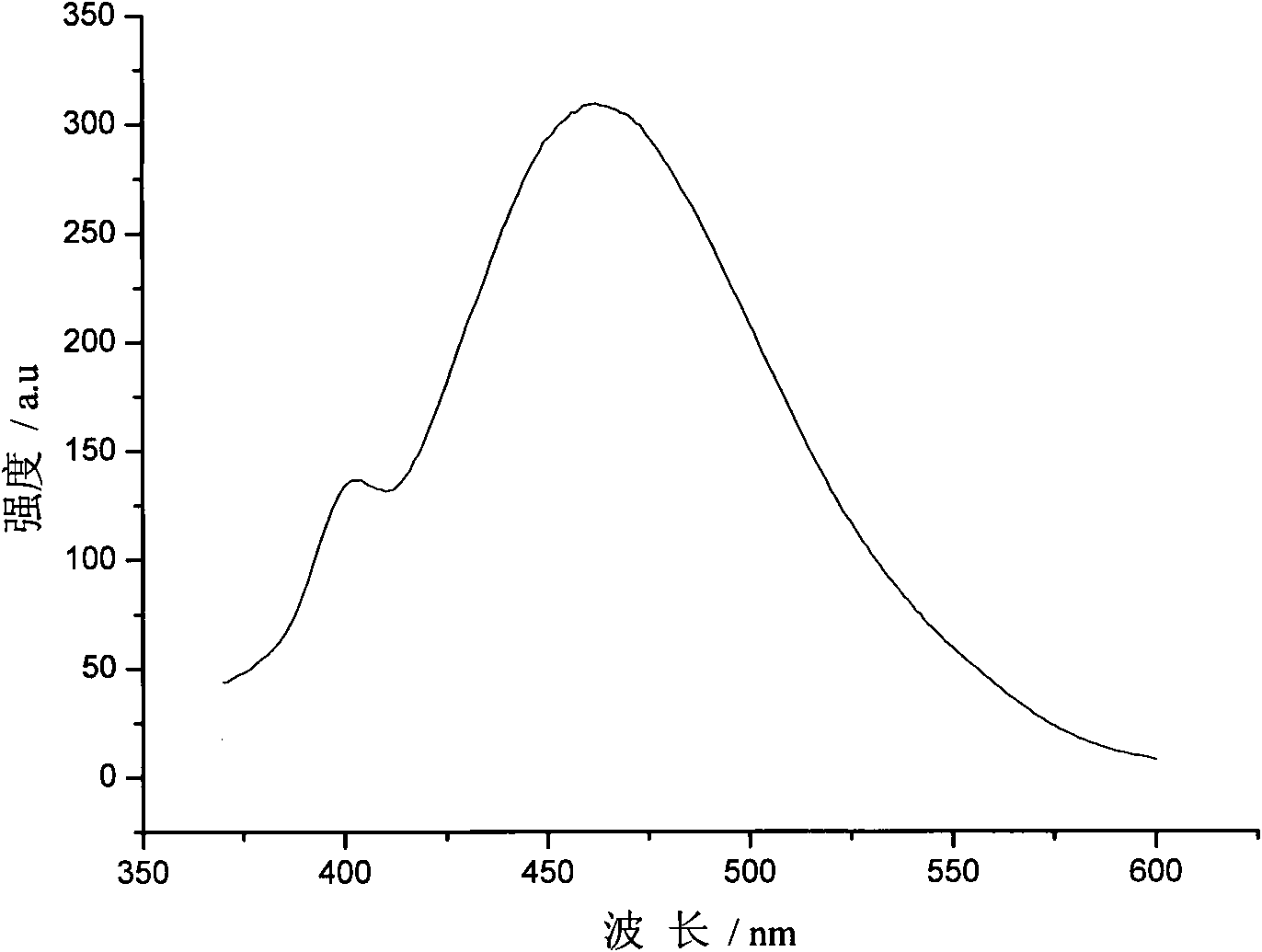
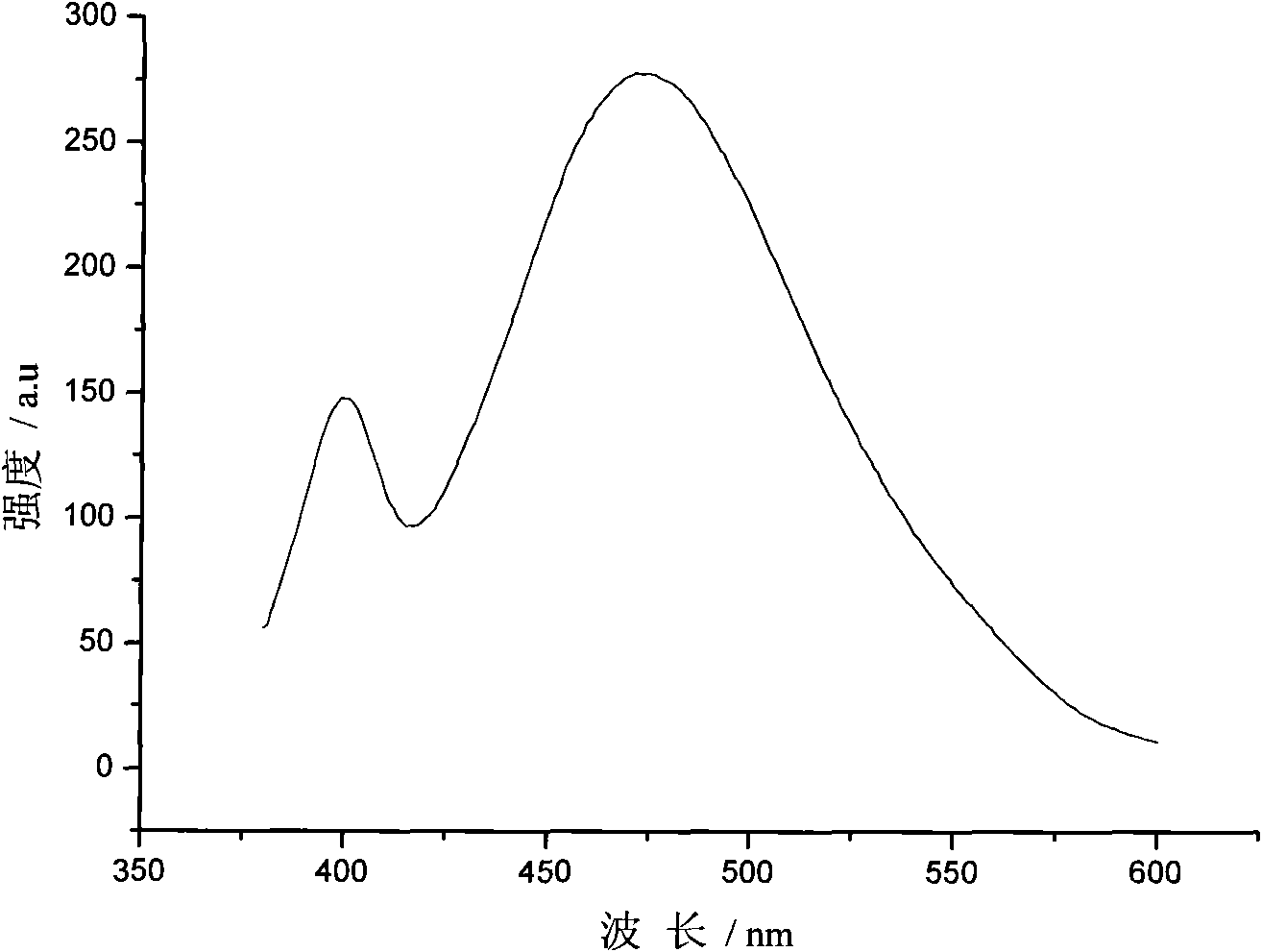
Preparation method of ZnSe-to-Cu quantum point
A quantum dot and content technology, which is applied in the field of ZnSe:Cu quantum dot preparation, can solve the problems of dangerous operation, high price, harsh conditions, etc., and achieve the effect of simple and safe operation and reduced preparation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] (1) Preparation of Se precursor
[0024] Under the protection of nitrogen, 0.007g of Se powder and 0.01g of sodium borohydride were dissolved in 3ml of distilled water, and heated appropriately to completely dissolve them to obtain a Se precursor solution.
[0025] (2) Preparation of Zn and Cu mixed solution
[0026] Dissolve 0.15g of zinc acetate and 0.0003g of copper acetate in 25ml of distilled water, add 54μl of thioglycolic acid, stir evenly with a magnetic force, and adjust the pH to 8.5 with 1mol / L NaOH to prepare a mixed solution of Zn and Cu.
[0027] (3) Synthesis of ZnSe:Cu quantum dots
[0028] Rapidly inject the Se precursor solution into the mixed solution of Zn and Cu, reflux the oil bath (90° C.), react for 1 hour, and cool to room temperature to obtain the ZnSe:Cu quantum dot aqueous solution. At room temperature, it can be directly used for spectral testing.
[0029] (4) Add 30ml of isopropanol to the ZnSe obtained above: Cu quantum dot aqueous solu...
Embodiment 2
[0031] (1) Preparation of Se precursor
[0032] Under the protection of inert gas argon, 0.006g of Se powder and 0.009g of sodium borohydride were dissolved in 2ml of distilled water, and heated appropriately to completely dissolve them to prepare a Se precursor solution.
[0033] (2) Preparation of Zn and Cu mixed solution
[0034] Dissolve 0.12g of zinc acetate and 0.0002g of copper acetate in 20ml of distilled water, add 48μl of thioglycolic acid, stir evenly with a magnetic force, and adjust the pH to 10.5 with 1mol / L NaOH to prepare a mixed solution of Zn and Cu.
[0035] (3) Synthesis of ZnSe:Cu quantum dots
[0036] Rapidly inject the Se precursor solution into the Zn precursor solution, reflux in an oil bath (100° C.), react for 1 hour, and cool to room temperature to obtain a ZnSe:Cu quantum dot aqueous solution. At room temperature, it can be directly used for spectral testing.
[0037] (4) Add 25ml of isopropanol to the ZnSe obtained above: Cu quantum dot aqueous...
Embodiment 3
[0039] (1) Preparation of Se precursor
[0040] Under the protection of inert gas nitrogen or argon, 0.01g of Se powder and 0.014g of sodium borohydride were dissolved in 3ml of distilled water, and heated appropriately to completely dissolve them to obtain a Se precursor solution.
[0041] (2) Preparation of Zn precursor
[0042] Dissolve 0.13g of zinc acetate and 0.0001g of copper acetate in 20ml of distilled water, add 50μl of mercaptoacetic acid, stir evenly with a magnetic force, adjust the pH to 9.0 with 1mol / L NaOH, and prepare a mixed solution of Zn and Cu.
[0043] (3) Synthesis of ZnSe:Cu quantum dots
[0044] Rapidly inject the Se precursor solution into the Zn precursor solution, reflux in an oil bath (100° C.), react for 2 hours, and cool to room temperature to obtain ZnSe quantum dot aqueous solutions with different reaction times. At room temperature, it can be directly used for spectral testing.
[0045] (4) Add 25ml of isopropanol to the ZnSe obtained above...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com