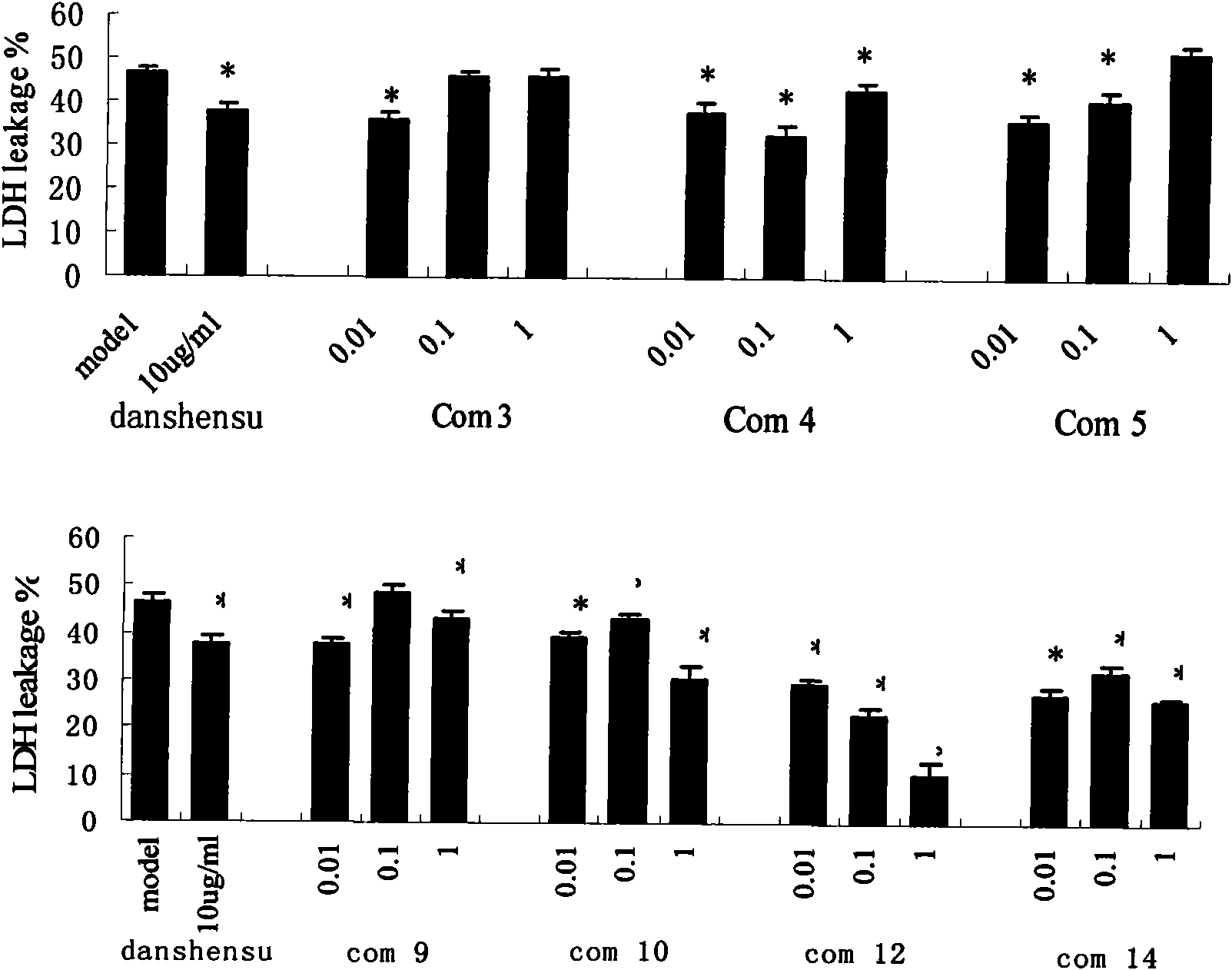
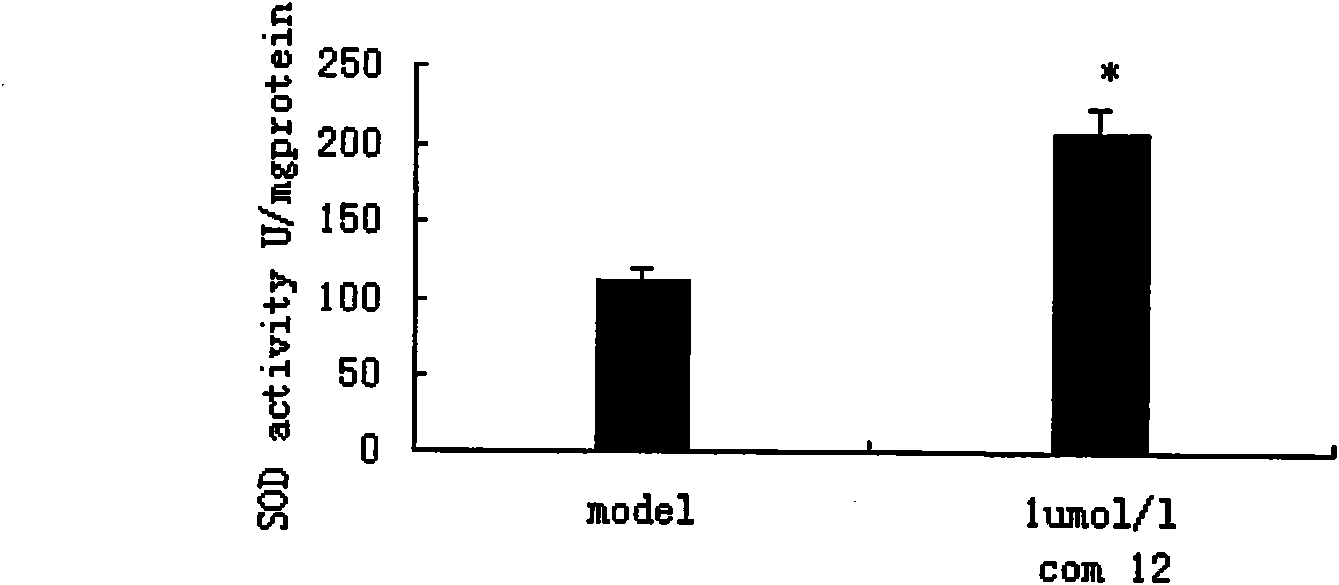
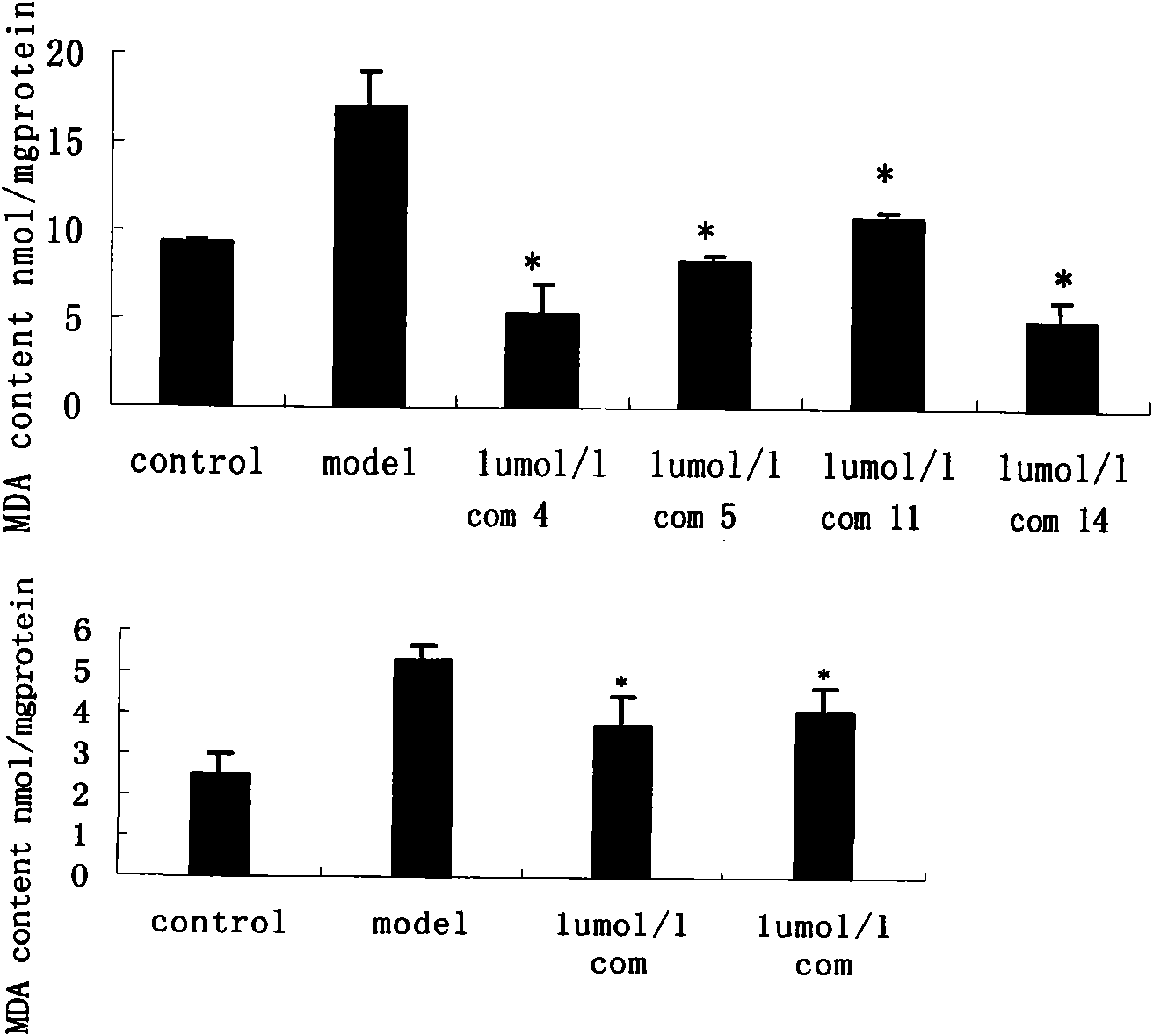
Danshensu derivatives and synthesis method and application thereof
A technology of danshensu derivatives and compounds, which is applied in the field of drug synthesis, and can solve problems such as unsuitable chiral resolution and chemical synthesis of d-danshensu and its derivatives that have not yet been seen.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] The preparation method of the present invention can be further embodied as follows with a representative compound preparation process:
[0034] Examples 1-6 of the present invention describe in detail the preparation of compounds 1-6 according to the method of scheme 1.
[0035] Examples 7-15 of the present invention describe in detail the preparation of compounds 7-14 according to the method of scheme 2.
Embodiment 1
[0036] Embodiment 1 According to flow process 1, when R is acetyl group,
[0037]
[0038] Synthesis of (Z)-2-acetylamino-3-(3,4-diacetoxyphenyl)acrylic acid (1):
[0039] Add 3,4-dihydroxybenzaldehyde 10.8g, 0.078mol and 30mL of anhydrous acetic anhydride, 32.4g, 0.319mol, anhydrous sodium acetate 6.4g, 0.078mol in a 250mL eggplant-shaped bottle, and react at room temperature for 3 hours to Petroleum ether / ethyl acetate (V / V=2:1) was the developing solvent, and TLC showed that the raw material point (R f =0.3) disappear and new point appears (R f =0.4), continue to add N-acetylglycine 9.2g in the reaction solution, 0.0789mol, heat to 100 ℃ and react for 2 hours, TLC shows the R of the product point f value is still 0.4, stop the reaction, the reaction solution is poured into 400mL cold water, overnight, a yellow solid precipitates, the solid is filtered and dissolved in ethyl acetate for recrystallization to obtain compound (1)(Z)-2-acetamido- 3-(3,4-diacetoxyphenyl)a...
Embodiment 2
[0040] Embodiment 2: Synthesis of (Z)-3-(3,4-dihydroxyphenyl)-2-hydroxyacrylic acid (2)
[0041] Add compound 1 (1g, 0.016mol) to 250mL eggplant-shaped bottle, add 9% hydrochloric acid 40mL, react under reflux conditions for 6 hours, the reaction solution is deep red, and chloroform / methanol / acetic acid (V / V / V=18 : 3: 1) as developing solvent, TLC showed raw material point (R f =0.9) disappear, there are new points (R f= 0.5) to generate, after dipping in phosphomolybdic acid, the new point can be baked into dark blue color, but the raw point cannot be baked in color, stop the reaction, wait for the reaction solution to cool, extract with ethyl acetate, dry the organic phase with anhydrous magnesium sulfate, filter , and evaporated to dryness under reduced pressure to obtain a dark red solid, which was washed with a small amount of water to obtain 0.53 g of a pale yellow solid of compound (2)(Z)-3-(3,4-dihydroxyphenyl)-2-hydroxyacrylic acid, with a yield of 87%. mp179-181℃ (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com